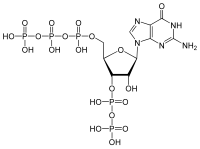
Guanosine 3 ′, 5 ′ bispyrophosphate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Guanosine 3 ′, 5 ′ bispyrophosphate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 10 H 17 N 5 O 17 P 4 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 603.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Guanosine-3 ', 5'-bispyrophosphate , or ppGpp is the signaling molecule of a bacterial stress response, the so-called stringent response . It is a derivative of guanosine diphosphate , which carries an additional pyrophosphate group on the 3 'atom of the ribose .
ppGpp was first discovered in Escherichia coli . In E. coli , ppGpp is an indicator of nutrient deficiency.
ppGpp cycle
In E. coli , the precursor molecule pppGpp is produced by two ppGpp synthetases from ATP and GTP , RelA and SpoT. RelA can also produce ppGpp directly. A 5'-phosphohydrolase cleaves the phosphate residue from pppGpp. RelA is bound to the ribosomes , acts as a sensor for unloaded tRNAs and synthesizes pppGpp when there is a lack of amino acids . SpoT is a cytosolic protein and synthesizes pppGpp when there is a glucose deficiency . In contrast to RelA, SpoT breaks down ppGpp to pyrophosphate and GDP. The DNA sequences of the relA and spoT genes of E. coli are similar, so they are paralogous genes. However , there are differences in the N-terminus , the so-called HD domain; which occurs in hydrolases is mutated in the relA gene. Therefore RelA cannot reduce ppGpp.
function
ppGpp binds to RNA polymerase and has a profound effect on the transcription of various genes. It reduces the rate of transcription of rRNA genes and induces the transcription of genes that are involved in amino acid biosynthesis. ppGpp is a global regulator of gene expression in E. coli.
ppGpp in other bacteria
In contrast to E. coli and many other bacteria, some bacteria, e.g. B. Bacillus subtilis and many other gram-positive bacteria, only via a single ppGpp metabolizing enzyme that makes and breaks down ppGpp. In many pathogenic bacteria, ppGpp plays an important role as a global regulator of gene expression. In these bacteria, ppGpp is even identified as a virulence factor :
- Mycobacterium tuberculosis
- Legionella pneumophila
- Pseudomonas aeruginosa
- Borrelia burgdorferi
- Vibrio cholerae
- Listeria monocytogenes
- different types of Brucella .
Thus, the ppGpp synthesis represents a previously unidentified, possible target for novel antibiotics . In Streptomyces coelicolor and other Streptomycetes , ppGpp is necessary for antibiotic biosynthesis.
In rhizobia , ppGpp is essential for the symbiosis between bacteria and plants and for nitrogen fixation . In archaea , ppGpp has not yet been detected.
ppGpp in plants
ppGpp is also found in plants. It is synthesized in the chloroplasts and also plays an important role in adapting to changed environmental conditions.
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ JD Keasling et al: Guanosine pentaphosphate phosphohydrolase of Escherichia coli is a long-chain exopolyphosphatase. In: PNAS . 90 (15), 1993, pp. 7029-7033. PMID 8394006 ; PMC 47069 (free full text, PDF).
- ↑ W. Haseltine, R. Block: Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. In: PNAS. 70 (5), 1973, pp. 1564-1568. PMID 4576025 ; PMC 433543 (free full text, PDF).
- ^ DR Gentry, M. Cashel: Subcellular localization of the Escherichia coli SpoT protein. In: J. Bacteriol. 177 (13), 1995, pp. 3890-3893. PMID 7601859 ; PMC 177113 (free full text, PDF).
- ^ VJ Hernandez, H. Bremer: Escherichia coli ppGpp-synthetase II activity requires spoT. In: J. Biol. Chem. . 266 (9), 1991, pp. 5991-5999. PMID 2005135 ; PDF (free full text access).
- ↑ KD Murray, H. Bremer: Control of spoT -dependent ppGpp synthesis and degradation in Escherichia coli. In: J. Mol. Biol. 259 (1), 1996, pp. 41-57. PMID 8648647 ; doi: 10.1006 / jmbi.1996.0300
- ↑ L. Aravind, EV Koonin: The HD domain defines a new superfamily of metal-dependent phosphohydrolases. In: Trends Biochem Sci . 23 (12), 1998, pp. 469-472. PMID 9868367 ; doi: 10.1016 / S0968-0004 (98) 01293-6 .
- ^ I. Artsimovitch et al .: Structural basis for transcription regulation by alarmone ppGpp. In: Cell . 117 (3), 2004, pp. 299-310. PMID 15109491 ; PDF (free full text access)
- ↑ LU Magnusson et al: ppGpp: a global regulator in Escherichia coli. In: Trends Microbiol. 13 (5), 2005, pp. 236-242. PMID 15866041 ; doi: 10.1016 / j.tim.2005.03.008 .
- ↑ G. Mittenhuber: Comparative genomics and evolution of genes encoding bacterial (p) ppGpp synthetases / hydrolases (the Rel, RelA and SpoT proteins). In: J. Mol. Microbiol. Biotechnol. 3 (4), 2001, pp. 585-600. PMID 11545276 .
- ↑ DF Warner, V. Mizrahi: Tuberculosis chemotherapy: the influence of bacillary stress and damage response pathways on drug efficacy. In: Clin. Microbiol. Rev. 19 (3), 2006, pp. 558-570. PMID 16847086 ; PDF (free full text access).
- ↑ AB Molofsky, MS Swanson: Differentiate to thrive: lessons from the Legionella pneumophila life cycle. In: Mol. Microbiol. 53 (1), 2004, pp. 29-40. PMID 15225301 .
- ↑ DL Erickson et al: Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. In: Infection and Immunity . 72 (10), 2004, pp. 5638-5645. PMID 15385461 ; PDF (free full text access).
- ↑ JV Bugrysheva et al: Borrelia burgdorferi rel is responsible for generation of guanosine-3'-diphosphate-5'-triphosphate and growth control. In: Infect. Immune. 73 (8), 2005, pp. 4972-4981. PMID 16041012 ; PDF (free full text access).
- ↑ S. Haralalka et al .: Mutation in the relA gene of Vibrio cholerae affects in vitro and in vivo expression of virulence factors. In: J. Bacteriol. 185 (16), 2003, pp. 4672-4682. PMID 12896985 ; PDF (free full text access).
- ↑ CM Taylor et al: Listeria monocytogenes relA and hpt mutants are impaired in surface-attached growth and virulence. In: J. Bacteriol. 184 (3), 2002, pp. 621-628. PMID 11790730 ; PDF (free full text access).
- ↑ M. DOZOT, RA Boigegrain, RM Delrue, R. Hallez, p Ouahrani-Bettache, I. Danese, JJ Letesson, X. De Bolle, S. Kohler: The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system VirB. In: Cell. Microbiol. 2006 PMID 16803581 .
- ↑ S. Kim, K. Watanabe, H. Suzuki, M. Watarai: Roles of "Brucella abortus" SpoT in morphological differentiation and intramacrophagic replication. In: Microbiology. 151, 2005, pp. 1607-1617. PMID 15870469 .
- ^ OH Martinez-Costa, P. Arias, NM Romero, V. Parro, RP Mellado, F. Malpartida: A relA / spoT homologous gene from Streptomyces coelicolor A3 (2) controls antibiotic biosynthetic genes. In: J. Biol. Chem. 271, 1996, pp. 10627-10634. PMID 8631867 .
- ↑ M. Moris, K. Braeken, E. Schoeters, C. Verreth, S. Beullens, J. Vanderleyden, J. Michiels: Effective symbiosis between Rhizobium etli and Phaseolus vulgaris requires the alarmone ppGpp. In: J. Bacteriol. 187, 2005, pp. 5460-5469. PMID 16030240 .
- ^ A. Calderon-Flores, G. Du Pont, A. Huerta-Saquero, H. Merchant-Larios, L. Servin-Gonzalez, S. Duran: The stringent response is required for amino acid and nitrate utilization, nod factor regulation, nodulation, and nitrogen fixation in Rhizobium etli . In: J. Bacteriol. 187, 2005, pp. 5075-5083. PMID 16030199 .
- ↑ K. Takahashi, K. Kasai, K. Ochi: Identification of the bacterial alarmone guanosine 5'-diphosphate 3'-diphosphate (ppGpp) in plants. In: Proc. Natl. Acad. Sci. UNITED STATES. 101, 2004, pp. 4320-4324. PMID 15010537 .