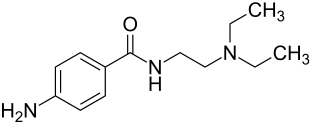
Procainamide
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Procainamide | |||||||||||||||||||||
| other names |
4-Amino- N - (2-diethylaminoethyl) -benzamide ( IUPAC ) |
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| boiling point |
210–215 ° C (267 Pa ) |
|||||||||||||||||||||
| pK s value |
9.32 |
|||||||||||||||||||||
| solubility |
Water: 5050 mg l −1 at 25 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Procainamide is structurally related to procaine . It is an antiarrhythmic in the group of sodium channel blockers. It belongs to the class Ia antiarrhythmics according to the Vaughan / Williams classification. Procainamide is used as a reserve antiarrhythmic for therapy-resistant ventricular arrhythmias and for supraventricular arrhythmias in pre-excitation .
history
Alfred Einhorn synthesized procaine in 1905 with the intention of discovering a new reserve therapeutic for cocaine in local anesthesia . The antiarrhythmic effect of procaine was not discovered until 1936 by Frederick Mautz in Cleveland , Ohio . However, because procaine hydrolyzed too quickly, it was unsuitable for clinical use. Lester Charles Mark (born 1918) studied the related procainamide, which was introduced in 1951 for the treatment of ventricular arrhythmias. Henry Max Woske (born 1924) also proved its effectiveness against supraventricular arrhythmias in 1953.
Pharmacokinetics
Procainamide has a short plasma half-life of 2 to 4 hours and a bioavailability of approximately 80%. Since procainamide is excreted via the kidneys , a dose adjustment should be considered in case of renal insufficiency .
Pharmacodynamics
It has a weaker anticholinergic effect than quinidine . Procainamide can induce anti-nuclear antibodies (systemic lupus erythematosus ) in long-term therapy , in which case it should be discontinued immediately. Furthermore, it is possible that treatment with procainamide can cause hives , fever and agranulocytosis .
Individual evidence
- ↑ a b entry on procainamide. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 1333, ISBN 978-0-911910-00-1 .
- ↑ a b c Entry on procainamide in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Data sheet Procainamide hydrochloride from Sigma-Aldrich , accessed on October 20, 2016 ( PDF ).
- ↑ Walter Sneader: Drug Discovery: A History . John Wiley & Sons, New York, NY 2005, ISBN 978-0-470-01552-0 , pp. 129 , urn : nbn: de: 101: 1-201412158328 .
- ^ Wolf-Dieter Müller-Jahncke , Christoph Friedrich , Ulrich Meyer: Medicinal history . 2., revised. and exp. Ed. Wiss. Verl.-Ges, Stuttgart 2005, ISBN 978-3-8047-2113-5 , pp. 163 .
literature
- Herold Internal Medicine 2004
- Karow / Lang Pharmacology and Toxicology 2003
- Hacketal / Oberdisse Pharmacology and Toxicology 1997
Web links
- Entry on procainamide at Vetpharm, accessed April 18, 2012.