Isobutane
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
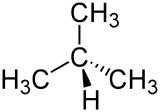 
|
|||||||||||||||||||
| Wedge formula (above) and skeletal formula | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Isobutane | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 4 H 10 | ||||||||||||||||||
| Brief description |
colorless gas with a sweet smell |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 58.12 g mol −1 | ||||||||||||||||||
| Physical state |
gaseous |
||||||||||||||||||
| density |
2.70 kg m −3 (at 0 ° C, 1013 hPa) |
||||||||||||||||||
| Melting point |
−159.42 ° C |
||||||||||||||||||
| boiling point |
−11.7 ° C |
||||||||||||||||||
| Vapor pressure |
301.9 k Pa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Dipole moment | |||||||||||||||||||
| Refractive index |
1.3518 (−25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
1000 ml m −3 |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Isobutane (systematic name according to IUPAC : methyl propane ) is an organic chemical compound from the group of alkanes . It is a constitutional isomer of n -butane and the simplest branched hydrocarbon .
Occurrence
Isobutane occurs in natural gases and petroleum . It is also obtained from it during cleaning and processing .
properties
Isobutane is a colorless, almost odorless, flammable gas . It is heavier than air and has a narcotic and suffocating effect in high concentrations. The flash point is −83 ° C, the ignition temperature is 460 ° C. The substance therefore falls into temperature class T1. For isobutane, the lower explosion limit (LEL) is 1.5% by volume (37 g / m 3 ) and the upper explosion limit (UEL) is 9.4% by volume (231 g / m 3 ). These values hardly differ from the values for the structural isomer n -butane. The limit oxygen concentration is 10.3% by volume.
use
Isobutane is a raw material in the chemical industry. Large quantities are used to produce alkylate ( alkylation , isooctane ). Some other chemicals, such as isobutene and tert-butyl hydroperoxide, are made from isobutane. Together with butane, it is used as a propellant in spray cans ( food additive E 943b). Since it is flammable like other alkanes, it is used as fuel. Isobutane is also used as an admixture for camping gas. Since isobutane has a lower boiling point than n -butane (−0.5 ° C), camping gas is mixed from propane and isobutane. This gas mixture is therefore not only suitable for summer, but also for use in winter and at full speed.
Refrigerant
Isobutane has a very low greenhouse effect ( GWP 3) and is therefore used in refrigerators and air conditioning systems as a refrigerant with the designation R-600a . For safety reasons, the application limit is currently 150 g per device. Since 1992 Greenpeace presented the Greenfreeze project with the help of Foron , R-600a has been used in refrigerators. In Europe, R-600a has almost completely replaced R-134a, which was previously used as a refrigerant in household appliances , as R-600a is cheaper, has a significantly lower global warming potential, has fewer chemical compatibility problems and is miscible with mineral oil . Most devices manage with less than 50 g of refrigerant. By 2011, 300 million devices had already come onto the market. Some solar collectors use methyl propane as a heat transfer medium.
Individual evidence
- ↑ Entry on E 943b: Isobutane in the European database for food additives, accessed on August 11, 2020.
- ↑ a b c d e f g h i j k l m Entry on isobutane in the GESTIS substance database of the IFA , accessed on December 21, 2019(JavaScript required) .
- ↑ Entry on butanes. In: Römpp Online . Georg Thieme Verlag, accessed on December 25, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Permittivity (Dielectric Constant) of Gases, pp. 6-188.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-308.
- ↑ Entry on isobutane in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Text of the additive approval regulation .
- ↑ greenpeace.de: Greenfreeze: The CFC-free refrigerator .

