s process
The s-process (s for slow , dt. Slow ) is one of the processes of nucleosynthesis .
procedure
The s-process is a neutron capture process which, in contrast to the fast r-process, takes place at low neutron densities and relatively low temperatures . It can synthesize elements up to a mass number A of 210, including particularly stable ones.
The s-process takes place mainly in stars that are located in the asymptotic giant branch of the Hertzsprung-Russell diagram . These are stars with a diameter of a thousand times the diameter of the sun , in whose core hydrogen and helium burning have already come to a standstill and in which helium is fused to carbon through shell burning in a shell around the core .
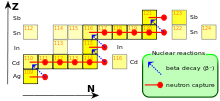
Fusion reactions also occur in these stars, releasing neutrons. Since neutrons (symbol n), unlike protons, have no electrical charge , they can penetrate to the atomic nucleus unhindered and accumulate there, releasing gamma quanta γ. This increases the mass number A and the neutron number N by 1 each, and a new isotope is created. The starting material of the s-process is primarily iron , which was present in the star from the beginning.
If an atomic nucleus becomes unstable after attachment due to a relative surplus of neutrons , a neutron becomes through radioactive β - decay , i.e. H. by sending out an electron e - and an electron antineutrino , converted into a proton. This creates the atom of another element with the same mass number, but an atomic number Z (proton number) increased by 1 and a neutron number N reduced by 1 ; the atom therefore takes the next higher place in the periodic table .
Due to the slow process of neutron accumulation, which extends over thousands of years, it is characteristic of the s-process that the β - decay of unstable isotopes takes place before another neutron is accumulated. As a result, all stable heavy elements can in principle be formed by it, but the proportion of heavier elements decreases drastically with increasing number of protons due to the low probability. Because of the relatively low neutron flux (on the order of 10 5 to 10 11 neutrons per cm² per second) that is expected during the s-process, the heavy, neutron-rich isotopes such as thorium and uranium can hardly be formed because the The initial nuclei required for this are generally subject to β - decay even before new neutron deposition. These isotopes are preferentially formed in the r process.
The s-process is often described mathematically by the local approximation , a theoretical model of the element abundances based on the assumption of a constant neutron flux in the star. The ratio of the element abundances is thus inversely proportional to the ratio of the effective cross-section of different isotopes for neutron capture. Because the larger this cross-section, the higher the probability of neutron capture and the associated conversion into another isotope.
The s-process ends with a cycle that leads back to the initial nucleus of the bismuth isotope 209 Bi (bismuth):
209 Bi + n → 210 Bi + γ (Neutron attachment) 210 bi → 210 Po + e - (β - decay) 210 Po → 206 Pb + 4 He ( α-decay ) 206 Pb + n → 207 Pb + γ (Neutron deposition 1) 207 Pb + n → 208 Pb + γ (Neutron deposition 2) 208 Pb + n → 209 Pb + γ (Neutron deposition 3) 209 Pb → 209 Bi + e - (β - decay)
Through the s-process, the stars of the asymptotic giant branch are the suppliers of half of the heavy elements beyond iron 56 Fe. The synthesized elements are transported outward by convection currents to the star's surface, where they can be detected spectroscopically . In 1952 the radioactive technetium in red giants was observed for the first time , which due to its half-life of a few million years could only have been formed shortly before by the s-process and thus supported the theory.
See also
literature
- Margaret Burbidge , Geoffrey Burbidge , William Alfred Fowler , Fred Hoyle : Synthesis of the Elements in Stars. In: Rev. Mod. Phys. 29th 1957, 547
- CE Rolfs, WS Rodney: Cauldrons in the Cosmos. Univ. of Chicago Press, 1988
- Heinz Oberhummer : Cores and Stars. Barth, 1993