foam
| Liquid foam |
| Hexagonal order and chaos in one foam |
| Solid foam: pumice stone |
Foam (from Middle High German schūm from Latin spuma ) are gaseous bubbles that are enclosed by solid or liquid walls. Foam used for fire fighting is called fire fighting foam . The formation of foam is called foaming .
Liquid foam walls
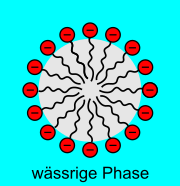
Liquid foam consists of small gas bubbles separated by liquid walls that are formed by surfactants and mostly water. Surfactants have two differently structured ends. One end is hydrophilic , which means "water-loving". In the following figures, these ends are marked with a minus sign or a red dot. The other end is hydrophobic (water-repellent) or lipophilic (fat-loving).
This structure makes the molecules surface-active, i. that is, they try to arrange themselves so that the hydrophobic part does not come into contact with water. This can be done in three main ways:
| (1) The molecules accumulate at the interface between water and air and lower the surface tension of the water. | (2) The molecules cluster together, form micelles and are thus “soluble” in water. | (3) If the liquid forms a thin film, the molecules accumulate in two flat layers. The hydrophilic ends protrude into the solution as in Fig. (1). |
- The surfactants accumulate at the interface between water and air. In this way you lower the interfacial tension . In the case of an area between the liquid and the gas phase, the interfacial tension is known as the surface tension .
- The surfactants can usually "dissolve" in water by agglomerating and forming small balls. The hydrophobic ends point towards the center. So-called micelles are formed . This means that surfactants are available “in reserve” in order to fill the new surface with molecules when the interfaces are enlarged.
- With surfactants, a water film with two surface layers can form more easily. The film is z. B. before a soap bubble . Here, too, the hydrophilic ends protrude into the aqueous phase.
The lowering of the surface tension of the aqueous phase through the accumulation of surfactants at the water / air interface means that air can be introduced into such a solution by whipping, blowing or similar methods and the air bubbles generated in this way are partially stabilized by the formation of a surface layer can without quickly coalescing . Foam is therefore a dispersion of air in a surfactant-containing solution, with a three-dimensional network of liquid lamellae enclosing the air as a continuous phase, thus forming polyhedra . This is why such foam is also called polyhedron foam .
| Formation of foam by air introduced. Spherical bubbles form when there is enough space between them and other bubbles. If the bubbles come close enough, they form common, almost flat contact surfaces (formation of polyhedra ). | Foam on the surface of a scanner . The polyhedron formation inside the foam is visible here. (See also here and Plateau's rules .) | Formation of a foam bubble , the " soap bubble ". Even in a film of water (interlamellar liquid) there are still "dissolved" surfactants. |
Depending on the structural and electrostatic properties of the surface-active molecules, foam bubbles with different sizes, wall thicknesses and lifetimes are created. In principle, the life of a liquid foam is limited. From a thermodynamic point of view, foams are metastable systems, since the overall system will strive to reduce the very large water / air interface to a minimum value. Due to the force of gravity, the interlamellar fluid slowly flows down between the foam bubbles. The wall becomes thinner and thinner in the upper area until it tears there.
Foam inhibitors and defoamers accelerate the breakdown of the foam. The Marangoni effect , on the other hand, helps stabilize foams under dynamic conditions.
In addition to the polyhedron foams described above , which only form in the presence of surfactants, there are also spherical foams . They consist of independent bubbles that may unite with one another when they come into contact. The service life of these foams depends on the toughness ( viscosity ) of the liquid. In low-viscosity liquids such as water, the foam disintegrates in seconds. (Example: opening a bottle with mineral water containing CO 2. )
Liquid foam issues
The formation of foam in liquids often causes problems in process engineering plants, since the foam penetrates into areas of the plant where the liquid should not reach. This is for example in the distilling off of solvents in the manufacture of synthetic resins , the case where the now gaseous solvent causes foaming of the resin. Defoamers are usually added here .
Two-dimensional foam
As a two-dimensional foam is called Polyederschäume where the polyhedron only are adjacent. To do this, the foam is compressed between two glass plates, so the bubbles are limited to two dimensions. The distance between the plates must be smaller than the diameter of the smallest bubble. Since there are no bubbles on top of each other, a two-dimensional foam can be easily observed.
The lamella runs straight between two neighboring cells with the same number of corners. If two neighboring cells have different numbers of corners, the lamella between them is curved, in the direction of the cell with the higher number of corners. In cells with a higher number of corners, there is therefore a lower air pressure . The Von Neumann law , describes how the size of the foam cells changes over time.
Solid foam walls
Solid foam consists of small gas bubbles that are separated by solid (i.e. non-liquid) walls. These include both elastically deformable sponges, such as a pot sponge , and only plastically deformable “hard” foams, such as the rigid foam board .
Examples:
- Pumice stone , a porous glassy volcanic rock, the specific weight of which is less than that of water.
- Foam , foam rubber , polystyrene as insulation and packaging material
- Assembly foam for assembly in construction (e.g. windows, doors)
- Rigid foam panels for thermal insulation in the building industry and as a partition wall in trade fair construction
- In-situ foam
- Foam glass
- Aerogels
- Metal foams , such as B. Aluminum foam for high-strength but light metal structures
- Foam concrete
- Sandwich foam for fiber composite materials (e.g. PVC rigid foam)
- Air chocolate
Classification in the scheme of chemical substances
| Schematic classification of the substances | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
- Crema (espresso)
- foam bath
- Sparkling wine
- Flotation , a physical separation process
- Vote
- Foam party
- Aphron
- Spray , meerschaum is the protein of dead algae that has been whipped up by wind and waves , for a chemical-physical explanation of the formation of meerschaum see egg white .
- Extinguishing foam