Schradan
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Schradan | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 24 N 4 O 3 P 2 | ||||||||||||||||||
| Brief description |
Deep brown, viscous liquid (technical product) |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 286.25 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.1343 g cm −3 |
||||||||||||||||||
| Melting point |
17 ° C |
||||||||||||||||||
| boiling point |
120-125 ° C (0.67 hPa ) |
||||||||||||||||||
| Vapor pressure |
0.13 Pa (25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Schradan is a chemical compound from the group of organophosphates that was used as an insecticide in the past .
presentation
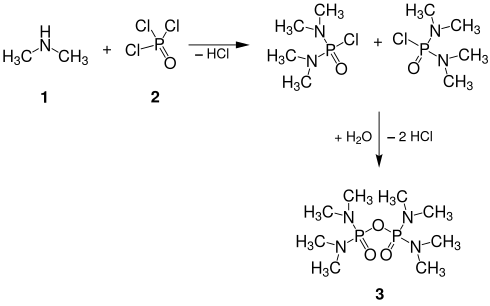
Schradan 3 can be prepared by reacting dimethylamine 1 with phosphorus oxychloride 2 and subsequent aqueous work-up.
etymology
The compound was named in honor of Gerhard Schrader , who first synthesized the compound in 1942.
properties
The compound is soluble in water, stable in air and only acts as an insecticide from concentrations above 0.1 M.
While the substance is highly toxic to mammals, it only has a toxic effect on some insect species. In order to be effective, however, the substance must first be metabolized. This occurs in mammals in vitro by oxidases in the liver - microsomes . According to this, the compound acts as an irreversible inhibitor of acetylcholine esterase . Antagonists of Schradans include Iproniazid and isoniazid .
Resistances
Resistance to Schradan was observed in the spider mites Panonychus citri and Tetranychus viennensis .
Individual evidence
- ↑ a b c Entry on Schradan in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on January 4, 2019.
- ↑ a b c d e f g Entry on Schradan in the GESTIS substance database of the IFA , accessed on January 4, 2019(JavaScript required) .
- ↑ Entry on Schradan in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on January 4, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Bhupinder Singh: NanoAgroceuticals & NanoPhytoChemicals . CRC Press , 2018, ISBN 978-1-351-13927-4 , pp. 83 ( limited preview in Google Book Search [accessed January 4, 2019]).
- ^ JE Gardiner, BA Kilby: Biochemistry of organic phosphorus insecticides . The mammalian metabolism of bis (dimethylamino) -phosphonous anhydride (Schradan). In: Biochemical Journal . tape 51 , no. 1 , April 1952, p. 78 , PMC 1197790 (free full text).
- ↑ Howard W. Chambers, Edward C. Meek, Janice E. Chambers: Hayes' Handbook of Pesticide Toxicology . Ed .: Robert Krieger. 3. Edition. Academic Press, 2010, ISBN 978-0-08-092201-0 , chap. 64 , p. 1395 ( limited preview in Google Book Search [accessed January 5, 2019]).
- ^ DF Heath: Organophosphorus Poisons . Anticholinesterases and Related Compounds. Pergamon Press, 1961 ( limited preview in Google Book Search [accessed January 4, 2019]).