Spray can
A spray can (also spray can or aerosol can ) is a metal can for spraying liquids such as hairspray , deodorant , shaving foam , paint , furniture polish, oil or even spray cream . These are under pressure as the propellants are propane , butane , dimethyl ether or mixtures thereof are used (where possible, compressed air or nitrogen ). Ozone harmful CFC propellants in spray cans are no longer used in Germany for two decades.
The ingredients of the spray cans can be sprayed out, atomized and applied through a fine nozzle. Depending on the application, an aerosol or foam is created immediately after the liquid emerges, depending on the amount of propellant gas , if the liquid does not form a small spray jet through coarser distribution . As a rule, the pressure is higher and the aerosol is finer than with the alternative pump nebulizer . Compared to other atomizers , spray cans offer a high spray performance with a small design. Since the propellant gases and usually the actual ingredients are flammable, they should not be brought into contact with flames , and aerosol cans should not be excessively heated, otherwise the gases contained may expand and burst the cans.
history
The engineer: Erik Andreas Rotheim
With his invention, the Norwegian engineer Erik Andreas Rotheim was actually responsible for the “birth” of the spray can. On October 9, 1927, he received a patent in Germany for “a method and device for spraying or distributing liquids or semi-liquid masses”. In doing so, he created the technical basis for all further developments of future generations. Originally looking for the best method for waxing his skis, he probably already suspected the wide range of other possible uses of his invention when he submitted the patent. In the first patent specification , he already listed a possible use of his pressurized gas packaging: “z. B. oils, fats , liquid soaps , resins , paraffins , types of wax , paints , paints, paints, varnishes , lacquers (e.g. cellulose lacquers), rubber , rubber , glue , disinfectants , impregnants , protective agents, cleaning agents , fertilizers , fire extinguishing agents , cosmetic preparations, organic and inorganic liquids ... "
The paint manufacturer: Richard Bjercke
In the first few years, the novelty initially stayed in Norway , which after the invention was also the scene of the first commercial production of spray cans. The paint manufacturer Richard Bjercke , who worked closely with Rotheim, initially produced paint and lacquer cans on a small scale together with Alf Bjercke and developed the technology further. Bjerckes' paint factory in Oslo was the largest production facility for paints and varnishes in Norway at the time.
The precision mechanic: Frode Mortensen
The precision mechanic Frode Mortensen was the third in the group of Norwegian tinkerers to take care of the "trimmings" and the correct valve technology for the new paint sprays. His patents on improved pressure vessels and optimized valves followed in 1938 and 1939.
The chemist: Lyle D. Goodhue
The chemist Lyle D. Goodhue had been looking for a suitable propellant and solvent that could be used to atomize insecticides since 1935 . Halogen compounds with a low boiling point were the focus of his research. These substances were also well suited as propellants because they were non-flammable and largely non-toxic.
The entomologist: William N. Sullivan
Together with entomologist (entomologist) William N. Sullivan, Goodhue tested adventurous methods of chemical nebulization. The tests of the various propellants repeatedly led to dead ends, until Goodhue remembered one of her first ideas and the work of Rotheim: Propellant No. 12, later known as Freon 12 , mixed with the required insecticide and in a valve-equipped pressurized gas cylinder Bottled according to the Rotheim principle, it brought the desired result: the legendary “bug bomb” (“insect bomb”) was born.
From then on, the aerosol can was extremely popular: first of all, starting in 1942, it saved the lives of countless American soldiers who fought not only against the Japanese in the Pacific War , but also against the malaria- transmitting Anopheles mosquito . After the end of the war, resourceful manufacturers increasingly took over and marketed the now extremely popular compressed gas innovation for their everyday products. Modified beer cans with plastic valves were the beginning of household mass products: the cans became more manageable, the containers lighter and the valves more cost-effective to manufacture. Especially during the so-called economic miracle in the mid-1950s, the modern spray can conquered private households. The cans were now made from lightweight aluminum or tinplate and also in much smaller and more consumer-friendly formats than before.
The first bestseller was hairspray around ten years later . The “liquid hairnet ” at the push of a button enabled women and their hairdressers to enjoy unimagined styling options and completely new hairstyle fashions from 1955 onwards . Since its inception, the hairstyle has been “anytime, anywhere,” as the advertising promised at the time. Or: "Whether wind, frost, rain - the spray makes your hair fit for any weather". Hairspray and deodorant make up the largest proportion of aerosol products these days . One product after the other has now found its way into the spray can: cosmetics , paints, household care products, but also medicines and food . There are only a few decades between the first post-war production in Kansas with 105,000 units in 1946 to the current production of several billion aerosol cans annually worldwide.
The inventor of the one-inch valve: Robert Abplanalp
The engineer Robert Abplanalp invented a valve (later called the one-inch valve) that made it possible to spray liquids that were in a can with the help of an added propellant. The valve could be mass-produced easily and cheaply. The previously far too great weight of the associated spray cans was decisively reduced by the use of the much lighter aluminum , so that now all possible substances in spray cans could be offered cheaply and easy to handle. The triumphant advance of the spray can began. Abplanalp patented his invention and founded the Precision Valve Corporation in Yonkers in 1949 with two partners . At the end of the 1950s Abplanalp bought the shares of the two co-founders and became the sole owner. Precision Valve Corporation became the leader in the world market. In 2004 the company provided approx. 4 billion spray cans per year and held over 300 patents in this production area. The harmfulness of CFC propellants ( chlorofluorocarbons ) - damage to the ozone layer and contribution to the greenhouse effect - was recognized in the 1980s and, according to international regulations, CFCs were essentially replaced by substances other than propellants.
Propellant
Early experimental spray cans used compressed air as a propellant. Since compressed air cannot be liquefied at room temperature, the propellant gas supply in these cans was correspondingly low. The propellant gas was usually exhausted rather than the useful content. That is why easily liquefiable gases are used as propellants, most of which are in liquid form in the can. In the past, dichlorodifluoromethane (R12, trade names Frigen and Freon ) was preferred . This gas was non-flammable, inert and non-toxic, so it was considered safe. Based on publications in 1974 and 1976, until 1985 ( Vienna Convention for the Protection of the Ozone Layer ) it was recognized that CFCs like dichlorodifluoromethane cause lasting damage to the earth's ozone layer. It was therefore replaced by other, less environmentally harmful propellants, which, however, have different disadvantages in use. Mixtures of lower alkanes such as propane (R290), n -butane (R600) and 2-methylpropane ( isobutane , R600a) are often used. Dimethyl ether and ethyl methyl ether are also used. These propellant gases are flammable and can form explosive mixtures with air. Carbon dioxide (R744) and nitrous oxide (laughing gas) are used for food . At room temperature, these propellant gases have a higher vapor pressure than the aforementioned propellant gases, which is why the aerosol cans have to be more robust, which is a cost disadvantage. 1,1,1,2-tetrafluoroethane (R134a) or 1,1,1,2,3,3,3-heptafluoropropane (R227ea) are used for medical products .
| Propellant | Abbreviation according to DIN 8960 | Molecular formula | molar mass (g / mol) | Melting point (° C) | Boiling point (° C) | Vapor pressure at 20 ° C (bar) | Vapor pressure at 30 ° C (bar) | Vapor pressure at 50 ° C (bar) | Ozone depletion potential ODP (R12 = 1) | Global warming potential GWP (CO 2 = 1) | Q |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethyl methyl ether | C 3 H 8 O | 60.1 | −139.2 | 7.4 | 1.601 | 2.3 | 4.1 | ||||
| n -Butane | R-600 | C 4 H 10 | 58.12 | −138.29 | −0.5 | 2.081 | 2.8 | 4.9 | 0 | 3 | |
| Isobutane (2-methylpropane) | R-600a | C 4 H 10 | 58.12 | −159.42 | −11.7 | 3.019 | 4.1 | 6.78 | 0 | 3 | |
| 1,1,1,2,3,3,3-heptafluoropropane | R-227ea | C 3 HF 7 | 170.03 | −131 | −17.3 | 3.993 | 9.16 | 0 | 2900 | ||
| Dimethyl ether | C 2 H 6 O | 46.07 | −141.5 | −24.82 | 5.102 | 6.9 | 11,431 | 0 | |||
| 1,1,1,2-tetrafluoroethane | R-134a | C 2 H 2 F 4 | 102.04 | −101 | −26 | 5.7 | 7.7 | 13.2 | 0 | 1300 | |
| Dichlorodifluoromethane | R-12 | CCl 2 F 2 | 120.91 | −157.8 | −29.8 | 5.7 | 7.5 | 12.2 | 1 | 8100 | |
| propane | R-290 | C 3 H 8 | 44.1 | −188 | −42 | 8.327 | 10.8 | 17.081 | 0 | 3 | |
| Nitrous oxide | N 2 O | 44.01 | −90.8 | −88.5 | 50.599 | 63.2 | ÜK | 298 | |||
| carbon dioxide | R-744 | CO 2 | 44.01 | −56.57 | −78.5 | 57.3 | 72.1 | ÜK | 0 | 1 |
ÜK ... supercritical. Critical temperature for N 2 O (T K / p K = 36.5 ° C / 72.7 bar) or CO 2 (31 ° C / 73.8 bar) exceeded, there is no longer a liquid phase.
Structure of a spray can
The basic component is usually a metal container, the actual “can” made of tinplate or aluminum . The bottom of this jar is curved inwards for several reasons:
- Arched forms are more pressure-resistant than flat bottoms, which would arch outwards under slight pressure,
- for safety reasons (if overpressure occurs due to the effects of strong heat, the floor can bulge outwards and thus provide pressure relief),
- for effective product use; the riser pipe reaching down to the inner edge of the can also reaches the last drop of the product,
- the spray can can be set up and therefore easier to use.
On top of the metal container are the valve, spray head and protective cap: the valve and spray head are responsible for “atomizing” the product and ensuring that it can be precisely metered. The spray head is provided with a protective cap (removable for some cans). The valve body is connected to a riser pipe that leads into the interior of the spray can. It reaches to the bottom and ensures that it is completely and evenly emptied. The gas phase inside the can also serves as an expansion space. This ensures that the filled spray can can withstand temperatures of up to 50 ° C.
Further indispensable components of the spray can are the liquid propellant or gas, because this generates the pressure required for spraying, and not least the actual product - the active ingredient that is to be sprayed. The latter is liquid and mixed with the propellant or gas in the can.
Aerosol cans made of tinplate or aluminum have to be formed in different molding and production processes, depending on the material. The cans are therefore produced by specialized companies.
Manufacture of spray cans from tinplate
Long sheet metal strips that weigh tons are the starting point for the manufacture of three-part tinplate spray cans. Production begins with the cutting of square “handy” panels, which are then printed: white paint, color printing and protective paint for the outer look, interior paint depending on the later filling to protect against corrosion. Spray paint cans with water-based paints in particular require strong protection against corrosion.
The body of the can is then cut out of the printed board, shaped into a cylinder and welded. A varnish or powder is then applied to protect the weld seam from corrosion. The lid and bottom of the can are also made separately from flat sheet metal. When flanging the top and bottom, these three parts are then firmly connected to one another by so-called hemming. A test for pressure stability and tightness completes the manufacturing process. In addition to the traditional manufacturing process for three-part tinplate cans, there is also a process for two-part tinplate cans, in which a bowl is drawn and ironed from a tinplate strip. The resulting can body is then crimped with the lid as with the three-part can.
Manufacture of spray cans from aluminum
Spray cans made of aluminum are seamlessly made from one piece. The starting material is aluminum strips. Circular disks (so-called slugs ) are punched out of this and shaped into raw cans in a press ( cold extrusion process ). In the further processing steps, the cans are washed, painted inside and out and then printed. Finally, the so-called shoulder and the valve seat are formed. Here, too, the production process concludes with the leak test of the finished can.
Filling the spray cans
All spray cans - whether tinplate or aluminum - are filled completely automatically and basically in the same way in the filling plant: After the can has been filled with the product, the valve is inserted and checked. Then the can is tightly closed with the valve disc by crimping . This creates a tight (homogeneous) connection between the can and the valve disc. Only then does the propellant gas fill, depending on the type of propellant. In the case of flammable propellants liquefied under pressure, such as propane / butane, the filling takes place in a separate, explosion-proof room. To be on the safe side, the spray can is never filled to 100 percent, because the propellant must be able to expand in the gaseous phase, in the "expansion space".
In the paint retail trade, spray cans are also used that are already filled with propellant but do not yet contain any paint. A previously mixed paint is then mechanically pressed into the spray can via the valve. This makes it possible to offer customers a variety of colors in spray cans without having to keep filled spray cans in various colors in stock.
Security check
The last step is a 100% safety check of the cans. Ready-to-use spray cans usually pass through a warm water test bath at 50 ° C. Due to the high temperature, the pressure in the can increases during this test. If a can were to leak, some of the contents would escape into the water and gas bubbles would immediately detect the leak. All faulty containers can thus be ejected. There are now alternative test methods that can sort out defective cans with the same reliability. In any case, the manufacturers guarantee that only pressure-resistant and leak-proof aerosol cans are packed and delivered.
Technical details
The spray can principle
Due to the internal pressure of the spray can, its contents are released as an aerosol when you press the spray head. The secret of this functionality lies in the mixture of active ingredient (the actual product) and liquid propellant inside the spray can: Part of the propellant is dissolved in the active ingredient and a second part is in gaseous form as a “pressure cushion” over the active ingredient-propellant mixture. When the spray button is actuated, the gaseous propellant pushes the contents out through the valve. At this moment the propellant evaporates in fractions of a second and the remaining active ingredient is distributed finely and evenly.
Two-chamber cans
Some active ingredients cannot or should not be easily mixed with a propellant inside the can - especially products that cannot be sprayed, such as pastes, gels or emulsions. By separating the ingredient and propellant, creamy or viscous substances such as B. shaving gels are automatically conveyed out of the can by actuating the valve.
The valve bag system is widespread in Germany. A coated aluminum bag connected to the valve is folded up and placed in the can. The can is filled with propellant and the valve is tightly connected to the can. Only then is the product filled into the bag. The propellant surrounds the product-filled bag like a cushion and exerts the necessary pressure for the removal of the product.
Another "two-chamber method" is the use of a piston in the can, which also separates the product from the pressure chamber. The Clayton piston is known from the USA, but there are always leaks there. In Europe, a German company offers a piston can with the patented ZIMA piston, which achieves high shut-off values with good long-term stability and allows the use of environmentally friendly propellant gases such as nitrogen, carbon dioxide or compressed air. Piston systems can make the cans easier to refill.
Two-chamber systems enable the can to be used in any position. In contrast to aerosol cans, they can be emptied regardless of whether the valve is at the top or at the bottom.
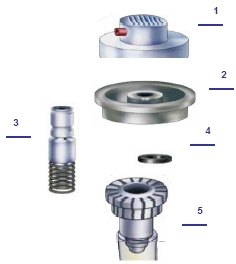
Valve technology
There are more parts and materials connected in a spray can valve than in the rest of the can. The starting point is a short plastic tube open at the top with a hole in the side (3). A rubber ring (4) around the tube seals the side hole. A spring is attached to the lower, closed side of the tube, which sits with the lower part of the tube in a plastic housing (5). The rubber ring rests on the edge of the housing. A metal bracket, the valve disc (2), presses this arrangement so tight that only the tube can move. If the tube on which the spray head (1) is attached is now pushed down, the rubber ring remains in its position and the small side hole is pushed under it into the inside of the can. At the same time, the spring is compressed in the housing. The pressure in the can causes the mixture of product and propellant gas to escape through the tube. If the pressure is no longer applied from above, the tube is brought up by the spring to its original position, and the rubber ring closes the hole on the side again. It is no longer spraying. The lower part of the housing and the plastic riser pipe are plugged into one another; the contents of the can is conveyed from the bottom of the can up to the valve through the riser pipe. Finally, a valve disc holds the valve components together with the spray can.
Spray properties
Valve technology is used to determine the spray properties of a product. Different spray patterns, each with special characteristics, are required for the different uses. One of the important characteristics is the droplet size, which determines how the sprayed product feels and what effect it achieves. When spraying hairspray, for example, very fine droplets are distributed without being seen. Such a very finely sprayed spray feels rather dry. If, on the other hand, large droplets are sprayed, you have a stronger wet effect. This is advantageous for all active ingredients in which a surface is to be slightly moistened evenly, such as. B. Furniture care. The droplet size is determined by various components:
- the ratio of active ingredient solution to propellant,
- the size of the valve opening,
- the size of the spray head opening.
These three influencing factors are coordinated in the manufacture of spray cans so that the droplet size is ideally suited for the application of the respective active ingredient / product. A shaving foam, for example, contains around five percent propellant, while a hairspray contains around 40 percent. The higher proportion of propellant ensures that the active ingredient solution or the actual product is broken down into smaller, finer droplets.
Spray paint cans
Spray paint cans consist of the following individual parts:
The spray paint can itself, then (see picture on the right):
- A - "Donut", plastic ring in the color of the filling
- B - valve system with riser pipe (detailed explanation see below)
- C - spray lock ring
- D - mixing balls (typical characteristic for spray paint cans)
- E - spray head
The valve is divided into the following components:
- Valve disc
- feather
- casing
- Riser pipe
The spring is especially important here, because its property determines the controllability of the aerosol flowing out during spraying. Modern valve systems from Belton and Montana have very soft springs, which is also noticeable because they are not that difficult to press and offer more "can control". This means that you can produce different line widths with one nozzle and one and the same can. In some modern cans, the riser pipe is "self-cleaning"; the instructions for use of the respective manufacturer must be observed. In the past, the cans should always be turned upside down after use and sprayed in order to empty the riser pipe.
The pressure of the can depends on the pressure of the filling. Cans specially made for fast and large-area spraying ("bombing cans") are under very high pressure and do not require mixing balls , art cans such as the Montana Gold , Sparvar or Belton Premium are filled with less pressure and therefore need balls to mix up the paint which separates from the solvents during storage and settles on the floor.
Nozzles ( caps )
The nozzle influences the spray pattern of the can. it consists of
- Version : lower part. z. B .: "full plate"
- Intermediate dosage pen: The pen that is inserted into the can. This pen usually contains a shaft or slot through which the lacquer gets into the cap.
- Vortex nozzle : The disc, visible from the front, with a small hole through which the paint exits the cap. The spray angle is controlled with this nozzle. Behind the window there is a small piston around which the paint flows.
Is the slot in the dosing pin z. B. very wide, so is the beam. If, however, the hole in the valve disc is also very large, the contents will only be injected, not sprayed, for example with shaving foam.
Disposal and recycling
disposal
After using the spray can, the question of proper disposal arises. The can itself is not classified as hazardous waste by the German Waste Catalog Ordinance (AVV) . The substances contained in the aerosol cans, on the other hand, sometimes have dangerous properties. If the spray cans are completely empty, they can be disposed of with the label Der Grüne Punkt via the yellow sack or the yellow bin . Incompletely emptied aerosol cans are to be taken to a pollutant collection point, since improper disposal of aerosol cans can lead to deflagrations, explosions and fires during storage, transport and processing of the material.
Recovery
Aerosol cans are made of aluminum and tinplate . After the used cans have been completely emptied, these materials are almost completely recycled and, after being crushed and melted down, processed into metal sheets. The gases or liquids contained therein are extracted and, as far as possible, recovered or disposed of in an environmentally friendly manner. The recycling of aerosol cans requires less energy than the production of new containers.
Web links
- Website of the Industry Association Aerosols e. V. (IGA) , the German professional association of the circles connected with spray cans (member companies are can and valve producers, fillers, propellant and solvent manufacturers, machine manufacturers etc.)
Individual evidence
- ↑ The spray can - a Norwegian achievement. In: DNFmagazin, issue 3/2016, p. 1 (www.dnfev.de)
- ^ National Academy of Sciences : Halocarbons, effects on stratospheric ozone . 1976. Retrieved October 23, 2013.
- ↑ Entry on ethyl methyl ether in the GESTIS substance database of the IFA , accessed on March 31, 2014 (JavaScript required)
- ↑ Entry on n-butane in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ^ Entry on isobutane in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ Entry on 1,1,1,2,3,3,3-heptafluoropropane in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ Entry on dimethyl ether in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ Entry on 1,1,1,2-tetrafluoroethane in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ Entry on dichlorodifluoromethane in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ^ Entry on propane in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ Entry on nitrous oxide in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ Entry on carbon dioxide in the GESTIS substance database of the IFA , accessed on March 31, 2014(JavaScript required) .
- ↑ https://de.wikibooks.org/wiki/Tabellensammlung_Chemie/_Dichte_gasförmiger_Stoffe
- ↑ Federal Environment Agency (UBA): Reading version of the regulation on the European list of waste. Retrieved November 13, 2019 .
- ↑ Der Grüne Punkt: What belongs in the yellow sack / yellow bin? Retrieved November 13, 2019 .
- ↑ Hazardous waste knowledge - the portal for hazardous waste: Danger from the can. Retrieved November 13, 2019 .
- ↑ Industriegemeinschaft Aerosole e. V .: The fascination of spray cans. Retrieved November 13, 2019 .
- ↑ Hazardous waste knowledge: danger from the can. Retrieved November 13, 2019 .
- ↑ Industriegemeinschaft Aerosole e. V .: The fascination of spray cans. Retrieved November 13, 2019 .