Swainsonine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
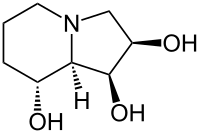
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Tridolgosir | |||||||||||||||||||||
| other names |
(1 S , 2 R , 8 R , 8a R ) -1,2,8-trihydroxyindolizidine |
|||||||||||||||||||||
| Molecular formula | C 8 H 15 NO 3 | |||||||||||||||||||||
| Brief description |
white crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 173.21 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
140-142 ° C |
|||||||||||||||||||||
| boiling point |
decomposition |
|||||||||||||||||||||
| solubility |
Easily soluble in water and ethanol |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Swainsonine is occurring in nature Indolizidine - alkaloid with strong pharmacological effect. Swainsonine reversibly inhibits the enzymes α- mannosidase in the lysosome and Golgi-α-mannosidase II of cells , which leads to an enrichment ( accumulation ) of mannose-rich oligosaccharides in the lysosome of these cells. In grazing animals that ingest swainsonine through their food, this can lead to so-called locoism , an induced mannosidosis .
Occurrence
- → see main article Locoweed
Swainsonine is the toxin in Locoweed . These are several types of plants and fungi that are found mainly in western North America. In grazing animals, the consumption of these plants leads to so-called locoism, a serious neurological disease.
biosynthesis
The biosynthesis of swainsonine has been studied in detail in the parasitic fungus Rhizoctonia leguminicola . L- saccharopene is initially formed from L- lysine . In the next stage, L -2-aminoadipic acid semialdehyde is formed , which cyclizes immediately to give Δ1-piperidine-6-carboxylic acid. Swainsonine is ultimately formed via the intermediate products ( S ) -pipecolic acid and 1-ketooctahydroindolizine .
Pharmacological potential
Swainsonine and some of its epimers show a strong pharmacological effect in animal experiments . The formation of metastases and tumor growth are suppressed. In addition, the formation of NK cells and the macrophage-mediated killing of tumor cells is stimulated. The proliferation of cells in the bone marrow is also activated. As an inhibitor of lysosmal α-mannosidase, the formation of tumor-specific glycosylation patterns is prevented and the catabolic glycosidases are inhibited. In animal experiments, both of these lead to reduced tumor growth and a reduction in the formation of metastases.
Swainsonine is currently in clinical trials . In a phase II study, however, no anti-tumor effect could be shown in the treated patients with advanced or metastatic renal cell carcinoma .
Swainsonine is an appetite suppressant.
The poisonous effect on grazing animals is based on the inhibition of the enzyme α-mannosidase . The symptoms of locoism are therefore similar to those of α-mannosidosis , a genetic disease . Swainsonine reversibly inhibits the enzymes α-mannosidase in the lysosome and Golgi-α-mannosidase II of cells , which leads to an accumulation of mannose-rich oligosaccharides in the lysosome.
discovery
Swainsonine was first isolated in 1973 from the fungus Rhizoctonia leguminicola and later from the eponymous legume Swainsona canescens and Astragalus lentiginosus .
further reading
- Gerhard Habermehl: Natural Products Chemistry. Springer, 2008, ISBN 978-3-540-73733-9 , p. 209 ( limited preview in the Google book search).
- JB Astroga et al .: Maternal ingestion of locoweed: II. The ability of intoxicated ewes to discriminate their own lamb. In: Small Ruminant Research 65, 2006, pp. 64-69, doi: 10.1016 / j.smallrumres.2005.05.026 .
- Y. Sichhart: Genes, enzymes and products of calystegin formation in Calystegia sepium (L.) R. Br. Dissertation, Martin Luther University Halle-Wittenberg, 2003.
- WG Armien: Comparative clinical and morphological investigations on spontaneous and experimental poisoning by Ipomoea fistulosa (Convolvulaceae) in goats. Dissertation, Justus Liebig University Giessen, 2000.
- MH Ralphs and LF James: Locoweed grazing. In: J Nat Toxins 8, 1999, pp. 47-51, PMID 10091127 .
- DR Tulsiani et al: Swainsonine induces the production of hybrid glycoproteins and accumulation of oligosaccharides in male reproductive tissues of the rat. In: Biol Reprod 43, 1990, pp. 130-138, PMID 2118392 .
- MD Skudlarek and MC Orgebin-Crist: Effect of swainsonine on rat epididymal glycosidases. (PDF; 655 kB) In: J Reprod Fert 84, 1988, pp. 611-617, PMID 3143832 .
- DR Tulsiani et al .: Production of hybrid glycoproteins and accumulation of oligosaccharides in the brain of sheep and pigs administered swainsonine or locoweed. In: Arch Biochem Biophys 264, 1988, pp. 607-617, PMID 3135781 .
- DR Tulsiani ua: The similar effects of swainsonine and locoweed on tissue glycosidases and oligosaccharides of the pig indicate that the alkaloid is the principle toxin responsible for the induction of locoism. In: Arch Biochem Biophys 232, 1984, pp. 76-85, PMID 6430242 .
- DR Tulsiani et al .: Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of golgi mannosidase II. In: J Biol Chem 257, 1982, pp. 7936-7939, PMID 6806288 .
Individual evidence
- ^ A b Duncan J. Wardrop, Edward G. Bowen: Nitrenium Ion-Mediated Alkenes Bis-Cyclofunctionalization: Total Synthesis of ( -) - Swainsonine . In: Organic Letters . tape 13 , no. 9 , 2011, p. 2376-2379 , doi : 10.1021 / ol2006117 .
- ↑ Yong-Shou Tian, Jae-Eun Joo, Bae-Soo Kong, Van-Thoai Pham, Kee-Young Lee, Won-Hun Ham: Asymmetric Synthesis of ( -) - Swainsonine . In: Journal of Organic Chemistry . tape 74 , no. 10 , 2009, p. 3962-3965 , doi : 10.1021 / jo802800d .
- ↑ Swainsonine at Santa Cruz Biotechnology, Inc., accessed January 8, 2012.
- ↑ a b Swainsonine data sheet from Sigma-Aldrich , accessed on April 23, 2011 ( PDF ).
- ^ PR Dorling et al.: Lysosomal storage in Swainsona spp. toxicosis: an induced mannosidosis. In: Neuropathol Appl Neurobiol 4, 1978, pp. 285-295, PMID 703929 .
- ↑ CM Harris et al: Biosynthesis of swainsonine in the Diablo locoweed (Astragalus oxyphysus). In: Tetrahedron Letters 29, 1988, pp. 4815-4818.
- ↑ a b c d G. Heimgärtner: Synthesis of polyhydroxylated indolizidine alkaloids and γ-amino acids. Dissertation, University of Regensburg, 2005.
- ^ AA Watson et al.: Polyhydroxylated alkaloids - natural occurrence and therapeutic applications. In: Phytochemistry 56, 2001, pp. 265-295, PMID 11243453 .
- ↑ K. Olden et al .: The potential importance of swainsonine in therapy for cancers and immunology. In: Pharmacol Ther 50, 1991, pp. 285-290, PMID 1754603 .
- ↑ JY Sun et al: Inhibition of the growth of human gastric carcinoma in vivo and in vitro by swainsonine. In: Phytomedicine 14, 2007, pp. 353-359, PMID 17097281 .
- ↑ JW Dennis et al .: Growth inhibition of human melanoma tumor xenografts in athymic nude mice by swainsonine. In: Cancer Res 50, 1990, pp. 1867-1872, PMID 2106389 .
- ↑ R. None: Calystegine in Solanum tuberosum L. - Cloning, expression and characterization of tropinone reductases I and II, putative enzymes of tropane alkaloid metabolism. (PDF; 85 kB) Dissertation, Martin Luther University Halle-Wittenberg, 2001.
- ↑ PE Shaheen et al .: Phase II study of the efficacy and safety of oral GD0039 in patients with locally advanced or metastatic renal cell carcinoma. In: Investigational New Drugs 23, 2005, pp. 577-581, PMID 16034517 .
- ↑ DH Pritchard et al: Swainsonine toxicosis suppresses appetite and retards growth in weanling rats. In: Research in Veterinary Science 48, 1990, pp. 228-230, PMID 2110378 .
- ↑ JL Ríos and PG Waterman: A review of the pharmacology and toxicology of Astragalus. In: Phytotherapy Research 11, 1997, pp. 411-418. doi : 10.1002 / (SICI) 1099-1573 (199709) 11: 6 <411 :: AID-PTR132> 3.0.CO; 2-6 .
- ↑ FP Guengerich include: Isolation and characterization of a l-pyrindines fungal alkaloid. In: JACS 95, 1973, pp. 2055-2056, doi: 10.1021 / ja00787a080 .
- ↑ SM Colegate et al.: A spectroscopic investigation of swainsonine: an α-mannosidase inhibitor isolated from Swainsona canescens. In: Aust J Chem 32, 1979, pp. 2257-2264, doi: 10.1071 / CH9792257 .
- ^ RJ Molyneux and LF James: Loco intoxication: indolizidine alkaloids of spotted locoweed (Astragalus lentiginosus). In: Science 216, 1982, pp. 190-191, PMID 6801763 .


