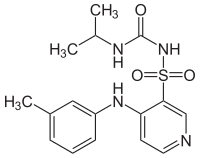
Torasemid
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Torasemid | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 16 H 20 N 4 O 3 S | |||||||||||||||||||||
| Brief description |
white to almost white, polymorphic powder |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 348.42 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
163-164 ° C |
|||||||||||||||||||||
| pK s value |
6.44 |
|||||||||||||||||||||
| solubility |
DMSO : 18 g · l −1 , insoluble in water, sparingly soluble in ethanol |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Torasemide is a drug from the group of loop diuretics that is used to treat high blood pressure , edema (water retention) or effusions due to heart failure .
It belongs to the group of water tablets ( diuretics ), which work on Henle's loop , part of the urine-producing system of the kidneys . These substances have in common the mechanism of action, by inhibiting a transport protein in the kidney tubules , causing a reduced reuptake of ions from the primary urine and thus leading to increased water excretion as a result of the change in osmotic pressure .
Clinical information
Application areas (indications)
Torasemide can be used in the therapy of high blood pressure and for the treatment or prevention of edema or effusion ( pleural effusion or ascites ) caused by heart failure .
Contraindications (contraindications)
Torasemid is allowed to
- Hypersensitivity to the active ingredient torasemide,
- Kidney failure with anuria ,
- Coma hepaticum or Praecoma hepaticum,
- Hypotension ,
- Hypovolemia ,
- Hyponatremia or hypokalemia
- Significant disruption of the urinary bladder emptying (for example, prostate enlargement of various causes ) and
- while breastfeeding
not be applied.
Drug interactions
Torasemid can increase the effect of ACE inhibitors and the side effects of digitalis preparations and reduce the effect of antidiabetic drugs. Probenecid and nonsteroidal anti-inflammatory drugs (NSAIDs) (such as acetylsalicylic acid and indomethacin ) can weaken the diuretic and blood pressure lowering effects of torasemid.
Use during pregnancy and breastfeeding
Torasemide may only be used during pregnancy if there is a compelling indication in the lowest effective dose. Its use is contraindicated during breastfeeding. If it is vital, it must be weaned .
Adverse effects (side effects)
Frequent (1–10%) are exacerbation of metabolic alkalosis , and increases in blood concentrations of uric acid , glucose , triglycerides and cholesterol . Hypokalaemia often occurs with a low-potassium diet, vomiting, diarrhea and after prolonged use of laxatives . Headache, dizziness, tiredness, weakness, loss of appetite, nausea, vomiting, diarrhea and constipation are particularly common at the start of treatment.
Comparison with furosemide and other loop diuretics
For the indication of heart failure, torasemide is an effective loop diuretic with a potency about 2.5 times that of furosemide . The diuretic effect seems to set in a little more slowly in lower doses and to last longer than with furosemide.
According to Arznei-Telegram , there is no evidence that torasemide has a more positive effect on mortality and symptoms than furosemide, and overall there are insufficient data, in particular no randomized studies, to compare the drugs. Furthermore, the manufacturer attracted attention with misleading advertising.
Other sources see distinct benefits to torasemide over furosemide in the treatment of heart failure.
Trade names
Monopreparations : Unat (A, D), Toracard (D), Toramid (CH), Torasem (CH), Torem (CH, D), numerous generics (D, A, CH)
Veterinary medicine: UpCard (D)
Individual evidence
- ↑ a b European Pharmacopoeia Commission (Ed.): EUROPEAN PHARMACOPOE 6TH EDITION . tape 6.0-6.3 , 2008.
- ↑ a b Entry on Torasemid. In: Römpp Online . Georg Thieme Verlag, accessed on November 10, 2014.
- ↑ a b c Torsemide data sheet at Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Entry on torasemide in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Assessment: Torasemid . Archived from the original on March 28, 2016. Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. Retrieved May 19, 2019.
- ^ GC Roush, R. Kaur, ME Ernst: Diuretics: a review and update. In: Journal of cardiovascular pharmacology and therapeutics. Volume 19, Number 1, January 2014, pp. 5-13, doi : 10.1177 / 1074248413497257 , PMID 24243991 (review).
- ↑ J. Buggey, RJ Mentz, B. Pitt, EL Eisenstein, KJ Anstrom, EJ Velazquez, CM O'Connor: A reappraisal of loop diuretic choice in heart failure patients. In: American Heart Journal . Volume 169, number 3, March 2015, pp. 323-333, doi : 10.1016 / y.ahj.2014.12.009 , PMID 25728721 , PMC 4346710 (free full text) (review).
- ^ RJ Mentz, V. Hasselblad, AD DeVore, M. Metra, AA Voors, PW Armstrong, JA Ezekowitz, WH Tang, PJ Schulte, KJ Anstrom, AF Hernandez, EJ Velazquez, CM O'Connor: Torsemide Versus Furosemide in Patients With Acute Heart Failure (from the ASCEND-HF Trial). In: The American journal of cardiology. Volume 117, number 3, February 2016, pp. 404-411, doi : 10.1016 / j.amjcard.2015.10.059 , PMID 26704029 , PMC 4718787 (free full text).
- ↑ ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9 , p. 222.
Web links
- MedlinePlus Druginfo (English)