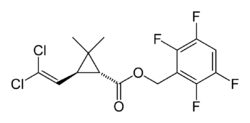
Transfluthrin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Transfluthrin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 15 H 12 Cl 2 F 4 O 2 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 371.16 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
32 ° C |
||||||||||||||||||
| solubility |
poor in water (57 μg L −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Transfluthrin is the trade name of a short-term insecticide developed by Bayer with a highly selective effect. It belongs to the pyrethroids and is a substituted carboxylic acid ester . Like all pyrethroids, transfluthrin unfolds its poisonous effect against insects due to their exposed nervous system and is therefore largely non-toxic for warm-blooded animals. According to the safety data sheet, inhalation represents practically no potential risk, but the substance is irritating to the eyes and skin. The half-life in soil and the environment is a few days.
Transfluthrin works equally against all insects, but also has a disgraceful effect and is therefore less ecologically relevant in typical local use. Transfluthrin is very toxic to fish and aquatic organisms and can have long-term harmful effects there.
Transfluthrin is commercially available in insect sprays, electric vaporizers and moth traps for private use (trade names: Bayothrin , Benfluthrin ). No pesticides containing transfluthrin are permitted in Germany.
Analytics
The reliable qualitative and quantitative determination of transfluthrin is possible after appropriate sample preparation by coupling gas chromatography with mass spectrometry .
Individual evidence
- ↑ a b c d e f Entry on Transfluthrin. In: Römpp Online . Georg Thieme Verlag, accessed on July 21, 2018.
- ↑ Entry on 2,3,5,6-tetrafluorobenzyl-trans-2- (2,2-dichlorovinyl) -3,3-dimethylcyclopropanecarboxylate in the GESTIS substance database of the IFA , accessed on February 1, 2016 (JavaScript required)
- ↑ Entry on 2,3,5,6-tetrafluorobenzyl trans-2- (2,2-dichlorovinyl) -3,3-dimethylcyclopropanecarboxylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016 . marketer can use the harmonized classification and labeling expand .
- ↑ Transfluthrin data sheet at Sigma-Aldrich , accessed on May 15, 2017 ( PDF ).
- ↑ a b Safety data sheet Transfluthrin from Scotts CELAFLOR GmbH & Co. KG, accessed on July 20, 2018.
- ↑ Kwan MWC, Weisenseel JP, Giel N, Bosak A, Batich CD, Willenberg BJ: Detection and quantification of trace airborne transfluthrin concentrations via air sampling and thermal desorption gas chromatography-mass spectrometry. , J Chromatogr A. 2018 Oct 26; 1573: 156-160, PMID 30224281

