Trimethylsilyl isothiocyanate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trimethylsilyl isothiocyanate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 9 NSSi | |||||||||||||||
| Brief description |
clear, light yellow liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 131.27 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density | ||||||||||||||||
| Melting point |
|
|||||||||||||||
| boiling point |
143 ° C |
|||||||||||||||
| solubility | ||||||||||||||||
| Refractive index |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Trimethylsilyl isothiocyanate can be understood as unstable isothiocyanic acid HNCS protected by the trimethylsilyl group - which is in tautomeric equilibrium with the likewise unstable thiocyanic acid HSCN. The compound is the starting material for thiocyanates and isothiocyanates , thioureas and nitrogen- and sulfur-containing (with the exception of 1,3,4-oxadiazole) heterocycles .
Manufacturing
Trimethylchlorosilane reacts with excess silver isothio-cyanate in inert solvents at 80 ° C. in approx. 85% crude yield to trimethylsilyl isothiocyanate. The reaction of anhydrous sodium thiocyanate NaSCN in a Soxhlet article with boiling chlorotrimethylsilane appears to be more advantageous in terms of preparation , in which trimethylisocyanatosilane is obtained in 78% yield after fractional distillation of the extraction mixture .
properties
Trimethylsilyl isothiocyanate is a clear, colorless to light yellow and unpleasantly pungent smelling liquid that reacts with water to decompose. Inconsistent isothiocyanic acid is formed with ethanol , which continues to react rapidly.
Applications
Thiocyanates and isothiocyanates
Trimethylsilyl isothiocyanate is suitable as a reagent for introducing thiocyanate and isothiocyanate groups as well as functional nitrogen-containing heterocycles.
Alkyl and aryl thiocyanates can be obtained under mild reaction conditions in sometimes very good yields (> 90%) by reacting TMSNCS with alkyl / aryl halides in the presence of the phase transfer catalyst tetrabutylammonium fluoride n-Bu 4 NF.
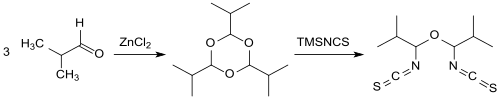
The implementation of TMSNCS with aldehydes , such as. B. isobutanal in the presence of catalytic amounts provides the Lewis acid , zinc chloride ZnCl 2 on the cyclic trioxane - trimer in high yield (89%) of an α, α'-di-isothiocyanatoether.
Nitrogen-containing five-membered heterocycles
In a variant of the Hantzsch thiazole synthesis , thioureas substituted with trimethylsilyl isothiocyanate and aromatic or primary and secondary aliphatic amines are formed, which can be cyclized with α-bromocarbonyl compounds to give 2-aminothiazoles. As a multicomponent reaction , this reaction can also be carried out by adding TMSNCS, the base triethylamine and an amine to an α-bromoketone in ethanol .
2-aminothiazoles are versatile synthetic building blocks for active pharmaceutical ingredients, such as. As the H 2 -receptor antagonist famotidine , the cephalosporin - antibiotic Cefdinir , the nonsteroidal anti-inflammatory drug meloxicam or the protein kinase inhibitor dasatinib .
3-Mercapto-1,2,4-triazoles are formed in the reaction of TMSNCS with carboxylic acid hydrazides via the intermediate stage of thiosemicarbazides through their cyclization using sodium hydroxide solution .
3-Mercapto-1,2,4-triazoles are building blocks for active ingredients with different pharmacological activities.
2-Amino-1,3,4-thiadiazoles can be obtained in high yields in a one-pot reaction of TMSNCS with acid hydrazides in ethanol in the presence of concentrated sulfuric acid .
2-Amino-1,3,4-thiadiazole is the basic structure for the carbonic anhydrase inhibitor acetazolamide , the nitroimidazole megazole against Chagas disease and African trypanosomiasis , as well as for antimicrobial substances with a different spectrum of activity.
Analogous to this are 2-amino-1,3,4-oxadiazoles by the one-pot reaction of TMSNCS with acid hydrazides in ethanol and the addition of an alkaline iodine / potassium iodide solution, the intermediate thiosemicarbazide being converted into the corresponding product with cyclization and sulfur elimination (cyclodesulfurization) in high yield Aminooxadiazole passes over.
Derivatives of 2-amino-1,3,4-oxadiazole also show diverse pharmacological properties.
Further reactions by TMSNCS to introduce the isocyanate group, e.g. B. in the conversion of alkenes into diisocyanates are reported.
Individual evidence
- ↑ a b c d e f g h data sheet trimethylsilyl isothiocyanate from Sigma-Aldrich , accessed on August 25, 2018 ( PDF ).
- ↑ a b Patent GB643941 : Improvements in and relating to organo-silicon compounds. Filed on September 27, 1950 , published on June 9, 1948 , Applicant: The British Thomson-Houston Co. Ltd ..
- ↑ a b c H.H. Anderson: Methyl silicon isothiocyanates. Molar refractions . In: J. Am. Chem. Soc. tape 69 , no. 12 , 1947, pp. 3049–3051 , doi : 10.1021 / ja01204a036 .
- ↑ RHCN Freitas: trimethylsilyl isothiocyanate (TMSNCS) . In: Austr. J. Chem. Band 69 , no. 8 , 2016, p. 928-929 , doi : 10.1071 / CH16057 .
- ↑ Patent GB643941 : Improvements in and relating to organo-silicon compounds. Filed on June 9, 1948 , published on September 27, 1950 , Applicant: The British Thomson-Houston Co. Ltd ..
- ↑ a b V. Golubev, F. Zubkov, M. Krasavin: A simple, three-component synthesis of 2-aminothiazoles using trimethylsilyl isothiocyanate . In: Tetrahedron Lett. tape 54 , 2013, p. 4844-4847 , doi : 10.1016 / j.tetlet.2013.06.102 .
- ↑ P.-Y. Renard, H. Schwebel, P. Vayron, E. Leclerc, S. Dias, C. Mioskowski: Optimized access to alkyl thiocyanates . In: Tetrahedron Lett. tape 42 , no. 48 , 2001, p. 8479-8481 , doi : 10.1016 / S0040-4039 (01) 01846-9 .
- ↑ K. Nishiyama, M. Oba: Reactions of trimethylsilyl isothiocyanate with aldehydes and acetals. Synthesis of symmetrically and unsymmetrically isothiocyanato-substituted ethers . In: Bull. Chem. Soc. Jpn. tape 60 , no. 6 , 1987, pp. 2289-2291 , doi : 10.1246 / bcsj.60.2289 .
- ↑ D. Das, P. Sikdar, M. Bairaqi: Recent developments of 2-amino-thiazoles in medicinal chemistry . In: Eur. J. Med. Chem. Volume 109 , 2016, p. 89-98 , doi : 10.1016 / j.ejmech.2015.12.022 .
- ↑ a b D.R. Guda, T. Wang, HM Cho, ME Lee: Trimethylsilyl isothiocyanate (TMSNCS): an efficient reagent for the one-pot synthesis of mercapto-1,2,4-triazoles . In: Tetrahedron Lett. tape 53 , no. 39 , 2012, p. 5238-5242 , doi : 10.1016 / j.tetlet.2012.07.054 .
- ↑ RM Shaker: The chemistry of mercapto- and thione-substituted 1,2,4-triazoles and their utility in heterocyclic synthesis . In: ARKIVOC . tape 9 , 2006, p. 59-112 , doi : 10.3998 / ark.55550190.0007.904 .
- ↑ DR Guda, HM Cho, ME Lee: Mild and convenient one-pot synthesis of 2-amino-1,3,4-thiadiazoles using trimethylsilyl isothiocyanate (TMSNCS) . In: RSC Adv. Band 3 , 2013, p. 6813-6816 , doi : 10.1039 / c3ra00159h .
- ↑ G. Serban, O. Stanasel, E. Serban, S. Bota: 2-Amino-1,3,4-thiadiazoles as a potential scaffold for promising antimicrobial agents . In: Drug Des. Dev. Ther. tape 12 , 2018, p. 1545-1566 , doi : 10.2147 / DDDT.S155958h .
- ↑ a b D.R. Guda, HM Cho, ME Lee: Mild and convenient one-pot synthesis of 2-amino-1,3,4-oxadiazoles promoted by trimethylsilyl isothiocyanate (TMSNCS) . In: RSC Adv. Band 3 , 2013, p. 7684-7687 , doi : 10.1039 / c3ra41044g .
- ↑ M. Bruno, R. Margarita, L. Parlanti, G. Piancatelli, M. Trifoni: Hypervalent iodine chemistry: Novel and direct thiocyanation of alkenes using [bis (acetoxy) iodo] benzene / trimethylsilyl isothiocyanate reagent combination. Synthesis of 1,2-dithiocyanates . In: Tetrahedron Lett. tape 39 , no. 22 , 1998, pp. 3847-3848 , doi : 10.1016 / S0040-4039 (98) 00629-7 .