Vamidothion
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
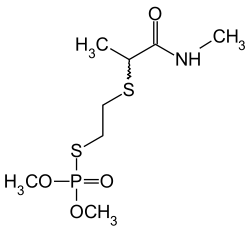
|
||||||||||||||||
| 1: 1 mixture of two stereoisomers | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Vamidothion | |||||||||||||||
| other names |
O , O -Dimethyl- S -5- ( N -methyl-2-methyl-3-thiavaleramide) thiophosphate |
|||||||||||||||
| Molecular formula | C 8 H 18 NO 4 PS 2 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 287.34 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
43 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Vamidothion is a mixture of two isomeric chemical compounds from the group of thiophosphoric acid esters . It is chiral and is used as an insecticide as a 1: 1 mixture of the mutually enantiomeric ( R ) form and the ( S ) form .
Extraction and presentation
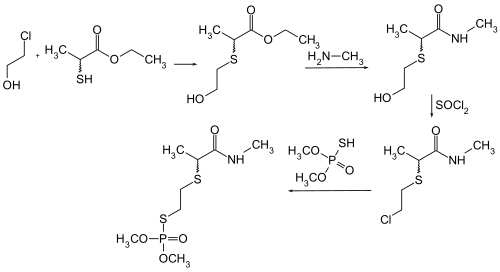
Vamidothion can be obtained by reacting 2-chloroethanol with ethyl 2-mercaptopropionate , methylamine , thionyl chloride and dimethyl thiophosphate .
properties
Vamidothion as a pure substance is a white crystalline solid, whereas the technical product is a yellowish, waxy solid. The technical product decomposes slowly at room temperature, but is stable in organic solvents (e.g. cyclohexanone , methyl ethyl ketone ). It hydrolyzes under alkaline conditions .
use
Vamidothion is used as an acaricide . The most important application is the use in apples and pears against aphids. It is also used on other pome fruits, sugar beets and, to a lesser extent, on grapes, grains, sugar cane and hops.
Admission
Vamidothion is not on the list of active ingredients for pesticides permitted in the European Union . In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c Joint Meeting on Pesticide Residues (JMPR), Monograph for Vamidothion , accessed December 9, 2014.
- ↑ a b c d e f Entry for CAS no. 2275-23-2 in the GESTIS substance database of the IFA , accessed on September 18, 2012(JavaScript required) .
- ↑ Entry on Vamidothion in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Vamidothion solution, 100 ng / μL in acetonitrile from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 338 ( limited preview in Google Book search).
- ↑ Regulation (EC) No. 2076/2002 (PDF) of the Commission of November 20, 2002.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Vamidothion in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved February 25, 2016.