mTOR
| MTOR | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 2549 amino acids | |
| Identifier | ||
| Gene name | FRAP1 | |
| External IDs | ||
| Orthologue | ||
| human | House mouse | |
| Entrez | 2475 | 56717 |
| Ensemble | ENSG00000198793 | ENSMUSG00000028991 |
| UniProt | P42345 | Q9JLN9 |
| Refseq (mRNA) | NM_004958 | NM_020009 |
| Refseq (protein) | NP_004949 | NP_064393 |
| Gene locus | Chr 1: 11.11 - 11.26 Mb | Chr 4: 148.45 - 148.56 Mb |
| PubMed search | 2475 |
56717
|
mTOR (abbr. for English. mechanistic Target of rapamycin , formerly mammalian target of rapamycin to German target of rapamycin in mammals ) is the name of all mammals occurring protein to which the immunosuppressant rapamycin binds indirectly. MTOR is an enzyme that is important for the survival, growth, proliferation and motility of cells . It adds a phosphate group to several other proteins and enzymes and activates them. Thus mTOR is part of the signal transduction in the body and the beginning of a cascade of signal pathways . Inhibition of mTOR is responsible for the immune-weakening effects of rapamycin.
The first molecular genetic studies were carried out in yeast in the early 1990s at the Biozentrum of the University of Basel , Switzerland, and by Sandoz Pharmaceuticals (now Novartis ) by Michael N. Hall , Joseph Heitman and Rao Movva. FKBP12, TOR1 and TOR2 were identified as targets for rapamycin. The researchers isolated rapamycin-resistant mutants of Saccharomyces cerevisiae and discovered that mutations in one of the three genes are responsible for the resistance. Two of the genes, TOR1 and TOR2, were designated as targets of rapamycin (target of rapamycin, TOR for short) - based on the Spalentor , a gate to the city of Basel and thus the place where TOR was first discovered. Michael Hall received the Louis Jeantet Prize for Medicine in 2009 for his work on TOR .
A few years later, in 1994, the Mammalian Target of Rapamycin (mTOR) was discovered by Solomon Snyder and David M. Sabatini at Johns Hopkins University and independently by Robert Abraham and Stuart L. Schreiber at Harvard University .
General properties
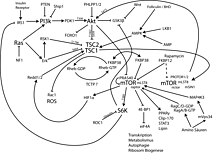
mTOR is part of a protein complex that integrates different signal pathways for growth factors , energy balance and oxygen concentration in the cell , regulates the translation of proteins and thus controls cell growth and the cell cycle .
mTOR was discovered while studying which proteins rapamycin binds to. mTOR consists of 2549 amino acids . The molecular mass is 290 kDa.
Activation of mTOR by stimulating growth factor receptors
If the growth factor - receptors by specific ligands stimulated (. Eg, IGF receptor), phosphorylated phosphoinositide 3-kinase (PI3K) , phosphatidylinositol 4,5-bisphosphate (PIP 2 ) to phosphatidylinositol 3,4,5-trisphosphate ( PIP 3 ). PIP 3 is a second messenger that causes other kinases such as PDK1 and protein kinase B (AKT) to bind to the membrane and be activated. The tumor suppressor phosphatase PTEN ( phosphatase and tensin homologue deleted on chromosome 10 ) abolishes the effect of PI3K by dephosphorylation of PIP 3 . The activated protein kinase B (AKT) phosphorylates and inhibits the Tuberous Sclerosis Complex (TSC) and thus eliminates its inhibitory influence on mTOR. TSC consists of two proteins, TSC1 ( hamartin ) and TSC2 ( tuberin ). TSC2 is a GTPase-activating protein (GAP) that with Ras related small GTPase Rheb ( Ras homolog enriched-in-brain ) inactivated by hydrolysis of GTP to GDP, which in turn activates mTOR.
Inhibition of mTOR due to lack of food
Energy depletion leads to a decrease in the concentrations of adenosine triphosphate (ATP) and amino acids in the cell and to the activation of serine threonine kinase 11 (STK11 or LKB1). LKB1 is a tumor suppressor protein that is inactivated in Peutz-Jeghers syndrome . LKB1 activates AMP-activated protein kinase (AMPK) . AMPK in turn phosphorylates and activates TSC2 and thus inhibits mTOR.
By integrating these two signaling pathways through Rheb and mTOR, cell growth (regulated by the growth factor PI3K-Akt pathway) is coordinated with the availability of energy and nutrients (regulated by the ATP-LKB1-TSC1 / 2 pathway).
Function of mTOR
mTOR exists in complexes with other proteins.
mTOR complex 1 (mTORC1) consists of mTOR, Raptor ( regulatory associated protein of mTOR ), mLST8 / GβL ( mammalian LST8 / G-protein β-subunit like protein ) and LST8 ( lethal with sec thirteen 8 )). mTORC1 is inhibited by rapamycin . Activation of mTORC1 leads to the phosphorylation of two key proteins that regulate the translation of proteins: 4E-BP1 ( eukaryotic initiation factor 4E (eIF-4E) binding protein-1 ) and S6K1 ( protein S6 kinase 1 ).
mTOR Complex 2 (mTORC2) consists of mTOR, Rictor ( rapamycin-insensitive companion of mTOR ), GβL, and mSIN1 ( mammalian stress-activated protein kinase interacting protein 1 ). mTORC2 is not inhibited by rapamycin. mTORC2 activates AKT by phosphorylation at the Ser473 position.
4E-BP-1 and S6K1
4E-BP1 and S6K1 are regulators of protein translation.
Unphosphorylated 4E-BP1 binds to the RNA-cap-binding protein eIF-4E and thereby inhibits the coupling to mRNA and the translation-initiation complex, which is required to initiate translation of cap-dependent mRNAs. Activated mTORC1 phosphorylates 4E-BP1, thereby releasing eIF-4E. This binds to cap mRNA transcripts and other proteins of the initiation complex, this binding initiates cap-dependent translation. The increased translation of cap-dependent mRNAs leads, among other things, to the synthesis of several proteins that control cell proliferation and regulate cell growth.
mTORC1 phosphorylates S6K1. This step stimulates the further phosphorylation of S6K1 by the master kinase PDK1 . Activated S6K1 stimulates the initiation of protein biosynthesis by activating the ribosomal protein S6 and other components of the translation machinery. In a positive feedback loop, S6K1 can phosphorylate mTORC1 and stimulate mTOR activity.
Medical importance
T cells , cells of blood and lymph vessels , smooth muscle cells and tumor cells are particularly sensitive to inhibition of mTOR .
In transplant medicine , the mTOR inhibitor rapamycin ( sirolimus ) is used to prevent rejection reactions . An advantage of rapamycin over other immunosuppressive drugs was seen in the lower incidence of tumors, but this could not be confirmed in large clinical studies. If rapamycin is used immediately after transplantation, however, the antiangiogenic and antiproliferative effects lead to increased wound healing disorders.
In cardiology , stents are inserted into closed or constricted coronary arteries to keep them open. The stent can become blocked due to the formation of new tissue. Coating the stents with rapamycin inhibits the formation of new tissue and lowers the rate of stent closure.
Temsirolimus , another mTOR inhibitor, improves survival in patients with advanced renal cell carcinoma . It is also approved for the treatment of the rare mantle cell lymphoma .
In hereditary cystic kidney disease , mTOR is upregulated in the epithelial cells of the kidney cysts. In the animal model, rapamycin leads to apoptosis of the cyst wall cells and thus inhibits the growth of the cysts.
While mTOR plays an important role in recovery and repair processes in acute kidney failure , in chronic kidney failure and diabetic nephropathy, permanent, inadequate activation of the mTOR signaling pathway contributes to the progression of kidney damage.
In patients with tuberous sclerosis and lymphangioleiomyomatosis , treatment with sirolimus leads to an improvement in the course of the disease.
| Glossary: | |
|---|---|
| 4E-BP1 | eukaryotic initiation factor 4E (eIF-4E) binding protein-1 |
| ACT | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| Cap | GTPase activating protein |
| GβL | mammalian LST8 / G-protein β-subunit like protein |
| IGF | Insulin-like growth factor |
| IGFR | Insulin-like growth factor receptor |
| LKB1 | Serine Threonine Kinase 11 (STK11) |
| mLST8 / GβL | mammalian LST8 / G-protein β-subunit like protein |
| mSIN1 | mammalian stress-activated protein kinase interacting protein 1 |
| mTOR | mammalian target of rapamycin |
| PDK1 | Phosphoinositide-dependent kinase 1 |
| PI3K | Phosphatidylinositol-3-kinase |
| PIP 2 | Phosphatidylinositol 4,5-bisphosphate |
| PIP 3 | Phosphatidylinositol 3,4,5-triphosphate |
| PTEN | Phosphatase and tensin homologue deleted on chromosome 10 |
| Raptor | regulatory associated protein of mTOR |
| Rheb | Ras-homolog-enriched-in-brain |
| Rictor | rapamycin-insensitive companion of mTOR |
| S6K1 | protein S6 kinase 1 |
| STK11 | Serine Threonine Kinase 11 (LKB1) |
| TSC | Tuberous Sclerosis Complex |
Individual evidence
- ↑ Heitman J, Movva NR, Hall MN .: Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast . In: Science . No. 253 , 1991, pp. 905-909 , PMID 1715094 .
- ^ N. Hay, N. Sonenberg: Upstream and downstream of mTOR . In: Genes Dev. No. 18 , 2004, p. 1926-1945 ( Article ). PMID 15314020
- ↑ Xuemin Wang, Christopher G. Proud: The mTOR Pathway in the control of protein synthesis . In: Physiology . No. 21 , 2006, p. 362-369 ( Article ).
- ↑ Janet E. Dancey: MTOR and Related Pathways . In: Cancer Biology & Therapy . No. 5 , 2006, p. 1065-1073 ( PDF ).
- ↑ R. Lowith et al .: Two TOR Complexes, Only One of Which is rapamycin-sensitive, Have Distinct Roles in Cell Growth Control. In: Molecular Cell . Vol. 10 (3), 2002, pp. 457-468 , PMID 12408816 .
- ↑ G. Blaeser-Kiel: Sirolimus for transplant protection. Better long-term prognosis due to lower tumor incidence . In: Deutsches Ärzteblatt . No. 104 , 2007, p. A-1255 ( article ).
- ↑ Henrik Ekberg et al .: Reduced Exposure to Calcineurin Inhibitors in Renal Transplantation . In: N Engl J Med . No. 357 , 2007, p. 2561-2575 ( abstract ).
- ↑ Gary Hudes et al .: Temsirolimus, interferon alfa, or Both for advanced renal cell carcinoma . In: N Engl J Med . No. 356 , 2007, p. 2271-2281 , PMID 17538086 .
- ^ W. Kühn, G. Walz: Autosomal dominant polycystic kidney disease . In: Dtsch Arztebl . No. 104 (44) , 2007, pp. 3022-3028 ( Article ).
- ↑ Wilfred Lieberthal, Jerrold S Levine: The role of the mammalian target of rapamycin (mTOR) in renal disease . In: Journal of the American Society of Nephrology . 20, No. 12, December 2009, pp. 2493-2502. doi : 10.1681 / ASN.2008111186 . PMID 19875810 .
- ↑ John J. Bissler et al .: Sirolimus for Angiomyolipoma in Tuberous Sclerosis Complex or Lymphangioleiomyomatosis . In: N Engl J Med . No. 358 , 2008, p. 140-151 ( abstract ).