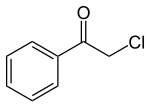
ω-chloroacetophenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | ω-chloroacetophenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 7 ClO | |||||||||||||||
| Brief description |
colorless to yellowish solid with an unpleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 154.59 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.32 g cm −3 |
|||||||||||||||
| Melting point |
56.5 ° C |
|||||||||||||||
| boiling point |
247 ° C |
|||||||||||||||
| Vapor pressure |
0.0054 mmHg (20 ° C) |
|||||||||||||||
| solubility |
slightly soluble in water (1.64 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.05 ml m −3 or 0.3 mg m −3 |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
ω-chloroacetophenone ( CN ) is a yellowish crystalline solid with an unpleasant odor. It was first made in 1871 by the German chemist Carl Graebe by chlorinating acetophenone .
use
ω-chloroacetophenone is used as an irritant in the form of aerosols , so-called tear gas . It was studied during the First and Second World Wars , but was never used. The United States of America used ω-chloroacetophenone in Vietnam . Its toxicity is apparently greater than that of CS and it has therefore largely been replaced by this. It was used for a long time in Germany in irritant gas cartridges for self-defense weapons.
CN is still available to paramilitaries and police forces in small, pressurized aerosol canisters known as "Chemical Mace" or tear gas. The can filling consists of 1.2% chloroacetophenone and a mixture of 1,1,1-trichloroethane and trichlorotrifluoroethane as well as hydrocarbons as a solvent and spray . In use, it has largely given way to pepper spray , as this works faster and spreads faster. However, it is still used as an admixture in water cannons .
As a chemical warfare agent , chloroacetophenone is outlawed under international law in the sense of the Geneva Protocol , which was confirmed again by the UN General Assembly in 1969 as a result of the deployment in Vietnam.
Effects
Like the CS gas, this compound acts in particular as an eye irritant and irritates the mucous membranes (in the mouth and nose area, the area of the bronchial system and the trachea and the conjunctiva ). Sometimes it can lead to more general reactions such as fainting and temporary loss of balance and orientation. Improper use can cause damage such as eye injuries and skin damage. Skin rashes and atopic eczema were observed as an allergic reaction to contact. In rare cases, deaths from lung damage have also been reported. Cases of skin cancer have also been reported.
literature
- Alfred Schrempf: Chemical Mace - How dangerous is chloroacetophenone? In: Chemistry in our time , 12th year 1978, No. 5, pp. 146-152, ISSN 0009-2851
- R. Klimmek, L. Szinicz, N. Weger: Chemical poisons and warfare agents. Hippokrates Verlag 1983, pp. 27 ff., ISBN 3-7773-0608-8
Individual evidence
- ↑ a b c d e f Entry on 2-chloroacetophenone in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d e Entry on omega-Chloroacetophenone in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on January 27, 2019.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 532-27-4 or ω-chloroacetophenone ), accessed on September 15, 2019.
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 74–75, ISBN 978-3-8348-1245-2 .
- ↑ POLICE: Outlawed internationally . In: Der Spiegel . No. 28 , 1981, pp. 51-52 ( Online - July 6, 1981 ).
- ↑ Entry on ω-chloroacetophenone. In: Römpp Online . Georg Thieme Verlag, accessed on August 5, 2016.


