1,3-indanedione
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,3-indanedione | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 6 O 2 | |||||||||||||||
| Brief description |
yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.14 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.37 g cm −3 |
|||||||||||||||
| Melting point |
129-132 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| pK s value |
7.2 (18 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,3-Indanedione is a diketone from the group of bicyclic aromatic hydrocarbons .
Extraction and presentation
1,3-indanedione can be obtained by a Claisen condensation with diethyl phthalate or dibutyl phthalate as starting material with subsequent hydrolysis and decarboxylation .
The synthesis of 1,3-indanedione is also possible by a reaction similar to the Diels-Alder reaction between o- xylylene and 4-cyclopentene-1,3-dione .
The oxidation of indane with oxidizing agents such. B. hydrogen peroxide or tert -butyl hydroperoxide only proceeds with poor yields, the main product being 1-indanone .
properties
Physical Properties
The enthalpy of formation of 1,3-indanedione in the gas phase at 298.15 K is −165.0 ± 2.6 kJ / mol, the enthalpy of fusion 17.2 kJ / mol and the enthalpy of vaporization 72.6 kJ / mol.
Chemical properties
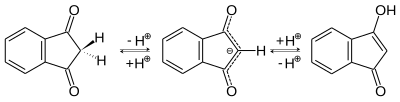
There is a keto-enol tautomerism in the 1,3-indanedione molecule
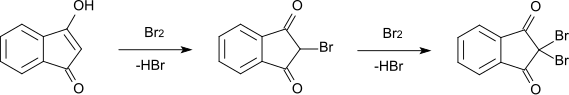
The bromination of 1,3-indanedione to 2-bromo-1,3-indanedione (melting point 118–120 ° C) takes place via the enol form with elimination of hydrogen bromide . A further bromination to 2,2-dibromo-1,3-indanedione (melting point 181–182 ° C) proceeds according to the same mechanism via the enol form of the monobromo derivative.
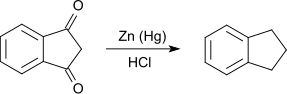
The reduction of 1,3-indanedione according to Clemmensen with amalgamated zinc in hydrochloric acid leads to indan. Indene is produced as a by-product .
Catalytic ionic hydrogenation with triethylsilane and trifluoroacetic acid also leads to indane.
If the reduction is carried out with sodium borohydride and palladium as a catalyst , the reduction only goes as far as 3-hydroxy-1-indanone or, subsequently, to 1,3-indanediol .
Reduction with zinc dust in glacial acetic acid also produces 3-hydroxy-1-indanone.
use
1,3-indanedione can be further processed to diphacinone (a rodenticide ) by reaction with 1,1-diphenylacetone .
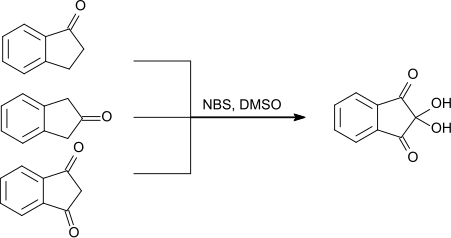
In addition to 1-indanone and 2-indanone, 1,3-indanedione can be used as a starting material for the production of ninhydrin . N -Bromosuccinimide and dimethyl sulfoxide are used as further reagents .
Related links
Individual evidence
- ↑ a b c data sheet 1,3-indanedione, 97% at AlfaAesar, accessed on December 1, 2019 ( PDF )(JavaScript required) .
- ↑ David R. Lide: CRC Handbook of Chemistry and Physics A Ready-reference Book of Chemical and Physical Data . CRC Press, 1995, ISBN 978-0-8493-0595-5 , pp. 330 ( limited preview in Google Book search).
- ↑ a b c data sheet 1,3-indandione, 97% from Sigma-Aldrich , accessed on December 1, 2019 ( PDF ).
- ↑ a b c M. A. Matos, MS Miranda, MJ Monte, LM Santos, VM Morais, JS Chickos, P. Umnahanant, JF Liebman: Calorimetric and computational study of indanones (PDF file; 102 kB), in: J. Phys. Chem. A , 2007 , 111 (43), pp. 11153-11159.
- ↑ CF Bernasconi, P. Paschalis: "Kinetics of ionization of 1,3-indandione in methyl sulfoxide-water mixtures. Solvent effect on intrinsic rates and Broensted coefficients", in: J. Am. Chem. Soc. , 1986 , 108 (11), pp. 2969-2977, doi : 10.1021 / ja00271a027 .
- ↑ CNKI: Synthesis of 1,3-indandione
- ↑ DB Hansen and MM Joullie : The development of novel ninhydrin analogues , in: Chem. Soc. Rev. , 2005 , 34 , pp. 408-417. doi : 10.1039 / b315496n
- ↑ J. Muzart: Homogeneous CrVI-Catalyzed Benzylic, Allylic and Propargylic Oxidations by tert-Butyl Hydroperoxide , in: Mini-Reviews in Organic Chemistry , 2009 , (6), pp. 9-20. doi : 10.2174 / 157019309787316120
- ^ A b D. Nematollahi, N. Akaberi: Electrochemical Study of Bromide in the Presence of 1,3-Indandione. Application to the Electrochemical Synthesis of Bromo Derivatives of 1,3-Indandione , in: Molecules , 2001 , 6 , pp. 639-646.
- ^ SA Galton, M. Kalafer, FM Beringer: Rearrangements in the Clemmensen reduction of 1-indanones and, 1,3-indandiones , in: J. Org. Chem. , 1970 , 35 (1), pp. 1-6. doi : 10.1021 / jo00826a001
- ^ OK Popova, ZN Parnes, MI Katinkin, SM Markosyan, NI Kopteva, LP Zalukaev, DN Kursanov: Ionic hydrogenation of 1,3-indanedione derivatives , in: Russian Chemical Bulletin , 1981 , 30 (9), pp. 1709-1711 . doi : 10.1007 / BF00949478
- ↑ JF Neumer: 2,3-Disubstituted 1-Indanones , United States Patent 3992450.
- ↑ SM Resnick, DS Torock, K. Lee, JM Brand, DT Gibson: Regiospecific and Stereoselective Hydroxylation of 1-Indanone and 2-Indanone by Naphthalene Dioxygenase and Toluene Dioxygenase (PDF file; 1.34 MB) in Applied and Environmental Microbiology , 1994 , 60 (9), pp. 3323-3328.
- ^ Thomas A. Unger: Pesticide Synthesis Handbook , Verlag William Andrew, 1996. ISBN 978-0-8155-1401-5 . P. 900 ( limited preview in Google Book search).
- ^ JL Hallman: Synthesis of Naphtho (f) ninhydrin and Synthesis of Polymer-supported Crown Ethers . Dissertation, 1991.