2- (4- tert- butylbenzyl) propionaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
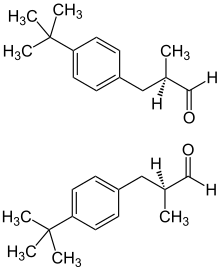
|
||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) |
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2- (4- tert- butylbenzyl) propionaldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 14 H 20 O | |||||||||||||||
| Brief description |
colorless liquid with a pleasant odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 204.31 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.946 g cm −3 |
|||||||||||||||
| boiling point |
279 ° C |
|||||||||||||||
| Refractive index |
1.505 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2- (4- tert- butylbenzyl) propionaldehyde or Lilial ® ( Givaudan ) is an aromatic aldehyde that is used in perfumes and fragrances and smells like lily of the valley . Since it is potentially allergenic, it must be listed on the packaging of the care products it contains, usually under the INCI name butylphenyl methylpropional .
properties
Structurally, Lilial is derived from Bourgeonal : Lilial has an additional methyl group on the C-2 atom. Lilial molecules have a chiral structure with a stereogenic center and thus come in two enantiomeric variants, ( R ) -Lilial and ( S ) -Lilial. The two enantiomers smell slightly different: ( R ) -Lilial smells more aldehydic-chemical while ( S ) -Lilial smells more flowery-oily.
synthesis
Lilial is manufactured on a large scale by BASF . In the first step, an acetal is produced from 4- tert- butyltoluene and methanol (CH 3 OH) by double anodic oxidation . This protected aldehyde function can then enter into a condensation reaction with propanal. The final step is a hydrogenation reaction.
Environmental relevance and dangers
The Scientific Committee on Consumer Safety (SCCS, scientific committee for consumer safety of the EU Commission) came to the conclusion in August 2015 that the use in washable (rinse-off) as well as in those that remain on the skin (leave-on) Cosmetics are "not safe".
2- (4- tert -Butylbenzyl) propionaldehyde was included in the EU's ongoing action plan ( CoRAP ) in 2012 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the uptake of 2- (4- tert- butylbenzyl) propionaldehyde were concerns about its classification as a CMR substance, consumer use and widespread use. The re-evaluation has been running since 2012 and is carried out by Sweden .
Individual evidence
- ↑ Entry on BUTYLPHENYL METHYLPROPIONAL in the CosIng database of the EU Commission, accessed on March 11, 2020.
- ↑ a b Entry on 2- (4-tert-butylbenzyl) propionaldehyde in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ a b c d e f data sheet 2- (4-tert-butylbenzyl) propionaldehyde from Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ List of the 26 allergens in cosmetics in the EU , accessed on May 9, 2018 (PDF; 11 kB).
- ↑ Regulation (EC) No. 1223/2009 of the European Parliament and of the Council of November 20, 2009 on cosmetic products .
- ↑ The Lilials. In: leffingwell.com. John C. Leffingwell, accessed May 9, 2018 .
- ↑ Cian Kingston, Maximilian D. Palkowitz, Yusuke Takahira, Julien C. Vantourout, Byron K. Peters: A Survival Guide for the “Electro-curious” . In: Accounts of Chemical Research . tape 53 , no. 1 , January 21, 2020, ISSN 0001-4842 , p. 72-83 , doi : 10.1021 / acs.accounts.9b00539 .
- ↑ Scientific Committee on Consumer Safety : OPINION ON Butylphenyl methylpropional (BMHCA). (PDF) March 16, 2016, accessed on August 1, 2017 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 2- (4-tert-butylbenzyl) propionaldehyde , accessed on March 26, 2019.



