2-butene-1,4-diol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
||||||||||||||||
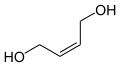
| cis -2-butene-1,4-diol (left) or trans -2-butene-1,4-diol (right) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | 2-butene-1,4-diol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 8 O 2 | |||||||||||||||
| Brief description |
colorless to yellowish odorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 88.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.075 g cm −3 |
|||||||||||||||
| Melting point |
10 ° C |
|||||||||||||||
| boiling point |
234-242 ° C |
|||||||||||||||
| Vapor pressure |
1.5 hPa (60 ° C) |
|||||||||||||||
| solubility |
miscible with water, ether and alcohol |
|||||||||||||||
| Refractive index |
1.445 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
2-butene-1,4-diol is a chemical compound from the group of diols .
Isomers
There are two isomeric 2-butene-1,4-diols, the ( Z ) - or cis - and the ( E ) - or trans -2-butene-1,4-diol. Without additional information, a mixture of isomers is meant.
| Isomers of 2-butene-1,4-diol | ||
| Surname | ( Z ) -2-butene-1,4-diol | ( E ) -2-butene-1,4-diol |
| other names | cis -2-butene-1,4-diol | trans -2-butene-1,4-diol |
| Structural formula |  |
|
| CAS number | 6117-80-2 | 821-11-4 |
| 110-64-5 (mixture of isomers) | ||
| EC number | 228-085-1 | 617-300-8 |
| ECHA info card | 100.025.532 | 100.132.685 |
| PubChem | 643790 | 175854 |
| Wikidata | Q27295224 | Q27237056 |
presentation
2-Butene-1,4-diol can be obtained by reducing 2-butyne-1,4-diol or by high-pressure synthesis from acetylene and formaldehyde . In the production from but-2-yn-1,4-diol, almost only cis- 2-butene-1,4-diol is formed.
The largest manufacturer of 2-butene-1,4-diol is ISP Marl GmbH, a subsidiary of Ashland .
properties
2-butene-1,4-diol is a slightly volatile, very difficult to ignite, colorless to yellowish odorless liquid that is miscible with water.
use
2-butene-1,4-diol is used in the manufacture of vitamin B6 , maleic acid , endosulfan , plasticizers and tetrahydrofuran .
Individual evidence
- ↑ a b c d e f g h i j k l m Entry on 2-butene-1,4-diol in the GESTIS substance database of the IFA , accessed on July 26, 2014(JavaScript required) .
- ↑ a b Entry on 2-butene-1,4-diol. In: Römpp Online . Georg Thieme Verlag, accessed on July 26, 2014.
- ^ Entry on 2-butene-1,4-diol in the Hazardous Substances Data Bank , accessed on July 26, 2014.

