Endosulfan
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
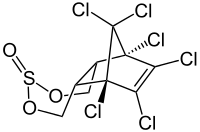
|
||||||||||||||||
| Mixture of three stereoisomers without indication of the relative stereochemistry at the sulfur atom | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Endosulfan | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 6 Cl 6 O 3 S | |||||||||||||||
| Brief description |
white crystals with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 406.93 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.75 g cm −3 (at 20 ° C) |
|||||||||||||||
| Melting point | ||||||||||||||||
| boiling point |
(Decomposition) |
|||||||||||||||
| solubility |
practically insoluble in water (0.33 mg l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 0.1 mg m −3 (measured as inhalable dust ) |
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Endosulfan is a neurotoxic insecticide from the group of bridged bicyclic compounds . It is a chlorinated hydrocarbon , which endogenously contains an inner ester of sulphurous acid (a sulfite ) as a functional group .
Manufacture and properties
Chemically, it is related to other cyclodiene pesticides such as aldrin , chlordane, and heptachlor . Similar to this, it is made from hexachlorocyclopentadiene via a Diels-Alder reaction (in the case of the endosulfan with cis - 2-butene-1,4-diol ) and subsequent esterification with thionyl chloride :
Technical endosulfan is a mixture of three stereoisomers . It consists of the two diastereomers α-endosulfan and β-endosulfan in a ratio of 7: 3, with α-endosulfan being present as a racemate . β-Endosulfan has a plane of symmetry, so it is a meso compound . The mechanism of the temperature-dependent irreversible isomerization of β-endosulfan into racemic α-endosulfan was investigated using spectroscopic methods.
The hydrolysis half-lives of α- and β-endosulfan at pH 7 are 19 and 11 days, respectively.
use
Endosulfan was developed as an insecticide in the early 1950s and approved by the North American Environmental Protection Agency (EPA) for the benefit of Hoechst AG in 1954 and then used in agriculture . Through the merger of Hoechst AG with Rhône-Poulenc SA in 1999 to form Aventis AG and the sale of the agrochemicals division Aventis CropScience to Bayer AG , Bayer CropScience was formed there, and the product endosulfan thus became part of the Bayer Group.
Endosulfan has been used in agriculture around the world to control insect pests such as whitefly , aphids , potato beetles and others. In addition to horticulture, it was also used in forestry and to combat tsetse flies . The World Health Organization estimates that annual world production in the early 1980s was around 9,000 tons .
The use of Endosulfan is prohibited in the European Union and in many other countries. However, it is still in use in countries like China and India . It has been banned in the US since 2010. It was owned by Bayer CropScience until 2007 and is currently manufactured by Makhteshim Agan and Hindustan Insecticides and marketed under the trade names Thiodan , Phaser and Benzoepine .
Because of the high toxicity and the ability to differentiate into organisms and the environment to enrich , in 2011 with the Stockholm Convention imposed a global ban.
In the United States, endosulfan was only approved for use in agriculture, where it was used in large quantities in the cultivation of cotton , potatoes and apples . The Environmental Protection Agency (EPA) estimates that around 700 tons of endosulfan were used between 1987 and 1997.
The connection was later withdrawn in stages:
- In 2000, the EPA withdrew its approval for private use.
- The United States Fish and Wildlife Service recommended that the EPA end the use of endosulfan in 2002. The EPA notes that young children between the ages of one and six are at risk of acute poisoning due to endosulfan residue in food. The EPA then restricted its use in agriculture, but approval remained until 2010.
- The international community took steps in 2007 to restrict the use and trade of endosulfan. In the Rotterdam Convention , endosulfan was listed as a single substance, among other things. The European Commission recommended inclusion on the list of prohibited chemicals in the Stockholm Convention . If possible, its use and manufacture should then be banned worldwide. Bayer CropScience withdrew endosulfan from the market in the United States. But other markets remained untouched.
- Several US agricultural associations and scientists called for the ban by the EPA in 2008.
- In 2010, traces of endosulfan were found in organic soy in southern Brazil.
- In April 2011, Endosulfan was included as a POP in Appendix A of the Stockholm Convention. This results in a worldwide ban on the manufacture and use of plant protection products, which came into force in 2012 with a transitional period of several years for certain crops. Admission was already planned for the Conference of the Parties in 2009, but failed at the time due to resistance from India.
safety instructions
Endosulfan was initially only classified as moderately toxic by the WHO ( LD 50 of 80 mg / kg). After recent studies showed LD 50 values between 18 and 355 mg / kg, the EPA and the WHO (from 1998) classified the compound as highly toxic. Various accidents with the connection resulted in water contamination, fish deaths and, especially in developing countries, human poisoning. The International Agency for Research on Cancer (IARC) has not classified endosulfan for its ability to cause cancer. However, some studies report a cancer-promoting effect. The compound (like DDT ) also has estrogenic properties.
toxicology
Endosulfan is a neurotoxin for insects (LD 50 of about 7 µg / honey bee), but also for mammals, including humans. The LD 50 value was determined to be 18–355 mg / kg. It acts as a GABA antagonist (γ-aminobutyric acid) on the transmitter-controlled chloride ion channel. In addition, it inhibits the Ca 2+ , Mg 2+ - ATPases . Both effects work on the transmission of information in the neuron. The symptoms of acute poisoning are hyperactivity, tremors, cramps, loss of coordination, shortness of breath, nausea and nausea. In severe cases, unconsciousness and death occur; Cases have been reported of death in humans after a dose less than 35 mg / kg.
Incidents
The Princess of the Stars ferry had ten tons of endosulfan on board when it fell into a typhoon off Sibuyan on June 22, 2008 and sank.
Fish deaths in the Middle Rhine in June 1969
On June 19, 1969, the introduction of the chemical into the Rhine led to extensive fish deaths in the Middle to the Lower Rhine (“from the helicopter, the river was silvery due to the fish carcasses floating on its stomach”). The cause could not yet be determined; a case by the public prosecutor's office was discontinued without result.
It was assumed that the contamination was due to the then producer Hoechst AG am Untermain or that some barrels of the very fish-toxic insecticide fell from a cargo ship into the Rhine at Binger Loch . The fish mortality was favored by the river water, which was quite warm due to a hot period, and by the high organic pollution of the Rhine at that time due to the very few sewage treatment plants, with a corresponding reduction in the oxygen content. This environmental catastrophe promptly led to a tightening of German water protection , the Water Management Act and the Wastewater Ordinance were changed from the softer "generally recognized rules of technology" that had previously been in force to the more stringent "state of the art".
Individual evidence
- ↑ a b c d e f g h Entry on endosulfan in the GESTIS substance database of the IFA , accessed on December 8, 2016(JavaScript required) .
- ^ A b Sylvan E. Forman, Antony J. Durbetaki, Michael V. Cohen, RA Olofson: Conformational Equilibria in Cyclic Sulfites and Sulfates. The Configurations and Conformations of the Two Isomeric Thiodans , J. Org. Chem. 30 (1965) 169-175, doi: 10.1021 / jo01012a039 .
- ↑ Entry on Endosulfan in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values , accessed on November 2, 2015.
- ^ G. Smith, CHL Kennard, KG Shields: Insecticides. XI. Crystal structure of endosulfan, β-6,7,8,9,10,10-hexachloro-1,5,5a, 6,9,9a-hexahydro-endo-6,9-methano-2,4,3-benzodioxathiepin 3-Oxide , Australian Journal of Chemistry 30 (1977), 911-916, doi: 10.1071 / CH9770911 .
- ^ A b Walter F. Schmidt, Cathleen J. Hapeman, Laura L. McConnell, Swati Mookherji, Clifford P. Rice, Julie K. Nguyen, Jianwei Qin, Hoyoung Lee, Kuanglin Chao, Moon S. Kim: Temperature-Dependent Raman Spectroscopic Evidence of and Molecular Mechanism for Irreversible Isomerization of β-Endosulfan to α-Endosulfan. In: Journal of Agricultural and Food Chemistry. 62 (2014), 2023-2030, doi: 10.1021 / jf404404w .
- ↑ Endosulfan - Draft Dossier prepared in support of a proposal of endosulfan to be considered as a candidate for inclusion in the UN-ECE LRTAP protocol on persistent organic pollutants , Umweltbundesamt, 2004, p. 29.
- ^ Agency of Toxic Substances and Disease Registry: Toxicological Profile for Endosulfan , 2000.
- ↑ Environmental Health Criteria (EHC) for Endosulfan , accessed November 29, 2014.
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Endosulfan in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 26, 2016.
- ↑ US EPA: EPA Action to Terminate Endosulfan .
- ↑ a b Umweltbundesamt: Endosulfan becomes dirty number 22 , press release.
- ^ Fifth Meeting of the Conference of the Parties to the Stockholm Convention: Endosulfan included under the Convention .
- ^ Benefits of Endosulfan in Agricultural Production: Analysis of Usage Information ( Memento of March 27, 2009 in the Internet Archive ) , US EPA, Docket ID NO. EPA-HQ-OPP-2002-0262-0062, 2007.
- ↑ US EPA: Reregistration Eligibility Decision for Endosulfan , November 2002.
- ↑ epa.gov: Reregistration Eligibility Decision For Endosulfan | NEPIS | US EPA , accessed February 27, 2017
- ^ A b Michael C. Newman: Fundamentals of Ecotoxicology The Science of Pollution, Fourth Edition . CRC Press, 2014, ISBN 978-1-4665-8229-3 , pp. 55 ( limited preview in Google Book search).
- ↑ epa.gov: Endosulfan Phase-out | Pesticides | US EPA , accessed February 27, 2017
- ↑ a b c Netherlands National Institute for Public Health and the Environment: Endosulfan. A closer look at the arguments against a worldwide phase out Letter report 601356002/2011 , MPM Janssen.
- ↑ ens-newswire.com: EPA: Pesticide Endosulfan Too Poisonous to Use , accessed February 27, 2017.
- ↑ CHEGA: Small farmers against pesticides .
- ↑ VDI Nachrichten: Long-lived poisons successfully banned , May 6, 2011.
- ↑ C. Vale, E. Fonfría, J. Bujons, A. Messeguer, E. Rodríguez-Farré, C. Suñol: The organochlorine pesticides gamma-hexachlorocyclohexane (lindane), alpha-endosulfan and dieldrin differentially interact with GABA (A) and glycine-gated chloride channels in primary cultures of cerebellar granule cells. In: Neuroscience. 117, 2003, pp. 397-403, PMID 12614680 .
- ↑ Poisons Information Monograph (PIM) for Endosulfan , accessed February 27, 2017.
- ↑ sueddeutsche.de : The sunken ferry had loaded tons of poison
- ↑ deutschlandradiokultur.de : 50 years ago: When water quality became a problem
- ↑ zeit.de : "The rats left the Rhine" , July 4th 1969.
- ↑ BBU-Wasserrundbrief No. 1145 of August 5, 2019 ( Federal Association of Citizens' Initiatives Environmental Protection ), p. 1.


