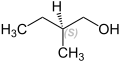2-methyl-1-butanol
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| Simplified structural formula without stereochemistry | ||||||||||
| General | ||||||||||
| Surname | 2-methyl-1-butanol | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 5 H 12 O | |||||||||
| Brief description |
colorless liquid with an unpleasant odor |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 88.15 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
0.82 g cm −3 |
|||||||||
| Melting point |
−70 ° C |
|||||||||
| boiling point |
129 ° C |
|||||||||
| Vapor pressure |
3.28 mbar (20 ° C) |
|||||||||
| solubility |
|
|||||||||
| Refractive index |
1.4107 (20 ° C, 589 nm) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||
2-Methyl-1-butanol is a chemical compound from the group of alcohols . It is one of eight structural isomers of pentanols . The compound occurs in two enantiomeric forms (or as a racemate ).
Occurrence
( S ) - (-) - 2-Methyl-1-butanol occurs naturally in practically all fruits (e.g. apples ), wine , beer and other spirits . It arises from the alcoholic fermentation as by-products from the amino acids leucine and isoleucine .
( R ) - (+) - 2-methyl-1-butanol can be detected as a component of the mold odor.
Extraction and presentation
( RS ) -2-methyl-1-butanol can be hydroformylated from n - butenes with a mixture of carbon monoxide and hydrogen in the presence of a cobalt hydrocarbonyl catalyst , a mixture of isomeric C5 aldehydes which are then hydrogenated to the associated amyl alcohol . A chlorination of pentanes is also possible.
( S ) - (-) - 2-methyl-1-butanol by fractionation of fusel oils recovered.
properties
( S ) - (-) - 2-Methyl-1-butanol is a flammable, volatile, colorless liquid with an unpleasant musty odor that is soluble in water. It has an odor threshold of 45 µg · m −3 .
use
( S ) - (-) - 2-Methyl-1-butanol is assigned to the Chiral Pool and is used in organic syntheses (introduction of an active amyl group ). In the USA it is used as a biochemical agent as a lure for hornets and wasps in traps.
safety instructions
The vapors of 2-methyl-1-butanol can form an explosive mixture with air ( flash point 40 ° C, ignition temperature 340 ° C). They also have a narcotic effect.
Web links
- ( RS ) -Racemat: Beilstein (System No. 24), Volume 1 H 388
- ( S ) - (-): Beilstein (Syst. No. 24), Volume 1 H 385
- ( R ) - (+): Beilstein (Syst. No. 24), Volume 1 H 388
Individual evidence
- ↑ a b c d e f g h i j Entry on 2-methyl-1-butanol in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Entry on (S) - (-) - 2-methyl-1-butanol at TCI Europe, accessed on April 2, 2012.
- ↑ a b Data sheet 2-methyl-1-butanol (PDF) from Merck , accessed on April 2, 2012.
- ↑ a b EPA: 2-Methyl-1-butanol (431602) Fact Sheet , accessed January 27, 2018.
- ↑ Günter Jeromin: Organic chemistry: A practical textbook . 2005, ISBN 978-3-8171-1732-1 , pp. 261 ( limited preview in Google Book search).
- ↑ Wolfgang Legrum: Fragrances, between stench and fragrance . Vieweg + Teubner, 2011, ISBN 978-3-8348-1245-2 , pp. 67 ( limited preview in Google Book search).
- ↑ a b c Entry on 2-methyl-1-butanol in the Hazardous Substances Data Bank , accessed on July 27, 2012.
- ↑ Wolfgang Mücke, Christa Lemmen: Bioaerosols and health . ecomed medicine, 2008, ISBN 978-3-609-16371-0 , p. 42 ( limited preview in Google Book search).