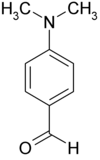
Dimethylaminobenzaldehyde
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Dimethylaminobenzaldehyde | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 11 NO | ||||||||||||||||||
| Brief description |
light yellow to green solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 149.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
72-75 ° C |
||||||||||||||||||
| boiling point |
176 ° C at 22.7 h Pa |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Dimethylaminobenzaldehyde , or more precisely, 4- ( N , N -dimethylamino) benzaldehyde is a benzaldehyde - derivative , which in para position a N , N transmits -Dimethylaminorest.
use
A solution of 2% dimethylaminobenzaldehyde in 20% hydrochloric acid is called an Ehrlich reagent or Ehrlich-Pröscher reagent . It is used to detect primary amino groups , pyrrole and indole derivatives. The Ehrlich reagent was named after the discoverer Paul Ehrlich .
In medicine , the Ehrlich reagent is used to detect porphobilinogen and urobilinogen in urine ( Watson-Schwartz test and Hoesch test ).
In pharmacy , the Ehrlich reagent is used to detect pyrrole and indole derivatives (e.g. ergot alkaloids ) ( Van-Urk reaction ).
In microbiology , dimethylaminobenzaldehyde ( Kovacs reagent ) is used to detect indole ( indole test ). Since it is also suitable for the detection of LSD in this application , it has achieved a certain level of awareness in the drug scene .
Individual evidence
- ↑ a b c d data sheet dimethylaminobenzaldehyde from Acros, accessed on February 19, 2010.
- ↑ a b Entry on dimethylaminobenzaldehyde in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Data sheet 4- (Dimethylamino) benzaldehyde from Sigma-Aldrich , accessed on May 5, 2011 ( PDF ).
- ^ S. Ebel and HJ Roth (editors): Lexikon der Pharmazie , Georg Thieme Verlag, 1987, p. 213, ISBN 3-13-672201-9 .
literature
- Pröscher, F. (1900): On the knowledge of Ehrlich's dimethylamidobenzaldehyde reaction . In: Hoppe-Seyler's journal for physiological chemistry . Vol. 31, pp. 520-526.