Aescin
Escin (also: Escin ) is an extract of the Horse-chestnut ( Aesculus hippocastanum ), and provides a mixture of more than 30 different saponins represent: FIG. Antiphlogistic properties.
composition
The main part makes up about 40% of the β-aescin . β-aescin, in turn, is a complex mixture which consists of more than 60% of the five pentacyclic triterpene sapogenins aescin Ia, Ib, IIa, IIb and IIIa. The aglyca the β-escins are di ester of Protoaescigenin and barringtogenol C in excess of the hydroxy group at C-3 with a branched triple sugar (Tri saccharide are linked). The composition of the trisaccharide varies between the respective aescin components.
The spontaneous migration of the acetyl group from C-22 to C-28 turns β-aescin into cryptoaescin. In contrast to β-aescin, cryptoaescin is hemolytically inactive and readily soluble in water.
A substance formerly known as α-escin is a mixture of the C-22 and C-28 acetates.
Structural variants of the Aescine
The structures of aescin Ia, Ib, IIa, IIb and IIIa are shown below.
The hydroxyl group of the C-21 atom in aescin Ia, IIa and IIIa is esterified with tiglic acid , in aescin Ib and IIb with angelica acid . In the aescins Ia and Ib, D - glucose is glycosidically linked via the C-2 'atom , in the aescins IIa and IIb D - xylose and in aescin IIIa D - galactose .
| Aescin | General structure | R 1 | R 2 | R 3 | Share in β-escin |
|---|---|---|---|---|---|
| Yes |

|
|
OH |

|
24% |
| Ib |
|
OH |

|
17% | |
| IIa |
|
OH |

|
13.5% | |
| IIb |
|
OH |

|
6% | |
| IIIa |
|
H |
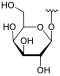
|
1.5% |
Medicinal use
| safety instructions | |||||||
|---|---|---|---|---|---|---|---|
| Surname |
Aescin Ia |
||||||
| CAS number |
6805-41-0 |
||||||
|
|||||||
| Toxicological data | |||||||
Aescin has a sealing effect on the vessel walls of the veins by changing their permeability. This is intended to reduce the flow of fluid into the tissue and accelerate the drainage of water deposits in the tissue (anti- exudative and edema protective effect). Mainly extracts standardized for aescin from the seeds of the horse chestnut ( adjusted horse chestnut seed dry extract ) or isolated aescin are used therapeutically . They are used to treat complaints of leg vein diseases ( chronic venous insufficiency ) as well as swelling after operations or sports injuries , hemorrhoids .
Trade names
Aesculaforce (CH), AesculaMed (CH), Aescuven (D), Essaven (D), Phlebostasin (CH), Reparil (A, D), Venostasin (D, A)
Demoven (CH), Opino (D, A), Reparil N (CH), Sportupac (D), Urelium (A)
Individual evidence
- ↑ Entry on Aescin. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b c E. Teuscher: Biogenic drugs. 5th edition, Wissenschaftliche Verlagsgesellschaft 1997, p. 159. ISBN 3-8047-1482-X
- ↑ Wagner, J. et al. (1970): About the ingredients of the horse chestnut. In: drug research . Vol. 2, pp. 205-209.
- ↑ Yoshikawa, M. et al. (1994) Escins-Ia, Ib, IIa, IIb, IIIa, bioactive Triterpene oligoglycosides from the seeds of Aesculus hippocastanum L .: their inhibitory effects on ethanol absorption and hypoglycemic activity on glucose tolerance test. In: Chem. Pharm. Bull. 42 (6), pp. 1357-1359; PMID 8069982 .
- ↑ Yoshikawa, M. et al. (1996): Bioactive Saponins and Glycosides. III. Horse chestnut. (1): The absorbent structures, inhibitory effects on ethanol, and hypoglycemic activity of escins Ia, Ib, IIa, IIb, and IIIa from the seeds of Aesculus hippocastanum L . In: Chem Pharm Bull (Tokyo) 44 (8); 1454-1464; PMID 8795266 .
- ↑ External identifiers or database links to Aescin Ia : CAS number: 123748-68-5, EC number: 683-254-0, ECHA InfoCard: 100.208.759 , PubChem : 6476030 , ChemSpider : 4977651 , Wikidata : Q72471092 .
- ↑ External identifiers of or database links for aescin Ib : CAS number: 26339-90-2, EC number: 247-619-4, ECHA InfoCard: 100.043.276 , PubChem : 6476031 , ChemSpider : 4977652 , Wikidata : Q27895506 .
- ↑ a b Data sheet Escin at Sigma-Aldrich , accessed on March 20, 2011 ( PDF ).
- ↑ Entry on aescin in the ChemIDplus database of the United States National Library of Medicine (NLM)
- ↑ Product information Venostasin retard 50 mg capsules, Astellas. As of July 2008.
- ↑ specialized information Reparil 40 mg coated tablets, Madaus . As of November 2007.
- ↑ ROTE LISTE 2017, Verlag Rote Liste Service GmbH, Frankfurt am Main, ISBN 978-3-946057-10-9 , p. 158.

