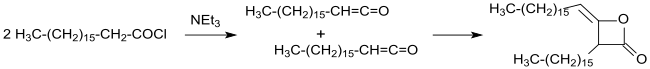Alkylated ketene dimers

Alkylated ketene dimers or alkyl ketene dimers (AKD) are based on the four-membered ring system of 2-oxetanone, which is also a central structural element of propiolactone and diketene . In oxetane ring of technically relevant alkylketene dimers a C located in position 3 of 12 C - 16 - alkyl group and in the 4-position a C 13 - C 17 - alkylidene .
The main application of alkylated ketene dimers is in bulk sizing - also known as sizing - of paper , cardboard and cardboard , as well as in the hydrophobization of cellulose fibers . The paper products modified in this way are distinguished by higher mechanical strengths and lower penetration by water, printing inks or inks.
history
Edgar Wedekind published the synthesis of alkyl ketene dimers in the reaction of carboxylic acid chlorides with tertiary amines as early as 1901 , but considered the reaction products to be polymers.
Since the molecular weight determinations of the early authors always pointed to a multiple of the atomic grouping R 1 R 2 CH = C = O, z. B. proposed a diketone structure with cyclobutane ring for the reaction product of isobutyryl chloride and triethylamine or postulated a so-called pyronone from propionyl chloride and tripropylamine .
The identification of the primary reaction products of carboxylic acid chlorides with α-hydrogen atoms with tertiary amines as ketenes by Hermann Staudinger and Norman Thomas Mortimer Wilsmore and the characterization of the ketenes as highly reactive compounds (ethenones), which in a dimerizing (2 + 2) cycloaddition 2- Forming oxetanones with a 4-position alkylidene group gradually clarified the constitution of the alkylated ketene dimers.
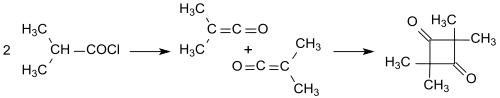
The elucidation of the constitution was made more difficult by the different dimerization products of ketenes. Thus, the simple ketene (H 2 C = C = O) dimerizes to diketene (4-methylene-2-oxetanone), while substituted ketenes, such as the dimethyl ketene (Me 2 C = C = O) formed from isobutyryl chloride with triethylamine , in one Head-to-tail addition to 2,2,4,4-tetramethyl-1,3-cyclobutanedione dimerizes.
The 2,2,4,4-tetramethyl-1,3-cyclobutanedione can easily be isomerized to dimethylketene dimer (4-isopropylidene-3,3-dimethyl-2-oxetanone).
The synthesis and characterization of hexadecyl ketene dimer, a key substance for AKD used in the paper industry, was first described in a patent in 1945 and in a publication in 1947.
A quantum chemical analysis excludes the formation of a cyclobutanedione in the dimerization of n-alkyl ketenes R-CH = C = O and favors the formation of the thermodynamically most stable 2-oxetanone structure.
Manufacturing
The industrial synthesis of alkylated ketene dimers - at that time still called ketoethenones - was started in 1945 from long-chain carboxylic acid chlorides in inert solvents, such as. B. Diethyl ether or benzene with triethylamine as a tertiary amine patented under anhydrous conditions. After filtering off the insoluble triethylamine hydrochloride and distilling off the solvent, long-chain alkyl ketene dimers are obtained in yields of up to over 90%.
The use of other solvents, such as. B. carboxylic acid esters or ketones for easier separation of the trialkylamine hydrochloride or other amines, such as. B. N, N, N ', N'-Tetramethyl-1,6-hexanediamine has no decisive advantages.
Processes without the use of solvents are also described, the amine hydrochloride formed either being filtered off or extracted with dilute aqueous acids.
A continuous process in which long chain carboxylic acid chloride and tertiary amine, e.g. B. dimethylisopropylamine, dimethylcyclohexylamine or triethylamine without solvent separated a tube reactor, a kneader or preferably a twin-screw extruder or planetary roller extruder fed, and is brought at temperatures between 90 and 110 ° C for reaction, yields in short reaction times Lactongehalte than 90%. The work-up is again carried out by phase separation or acidic extraction.
use
Alkylated ketene dimers as paper sizes
The problems with the acidic ( aluminum sulfate- mediated) sizing of paper with alkaline-digested rosin resins (see also paper disintegration ) led to the use of alkaline flocculants, such as. B. chalk or calcium carbonate as so-called alkali reserve to search for alternative materials for sizing in a neutral or alkaline environment. In addition to the much more reactive alkenylsuccinic anhydrides, which hydrolyze rapidly in the presence of water
the alkylated ketene dimers - starting in the 1950s - established themselves as the preferred surface and engine sizes in the paper industry from the 1960s.
AKD used in industry are derived from fatty acids with chain lengths between C 14 ( myristic acid ) to C 22 ( behenic acid ); Palmityl (C 16 ) diketene and stearyl (C 18 ) ketene and their mixtures, as well as fatty acid mixtures from the hydrolysis of animal and vegetable fats in the given chain length distribution are preferably used. Because of the chain length of the original fatty acids, AKDs are waxy solids with melting points between 42 and approx. 70 ° C. H. Mixtures of alkylated ketene dimers and water are dispersions at temperatures below 40.degree . C. and emulsions at temperatures above 45.degree .
Liquid AKD based on unsaturated fatty acids, such as B. oleic acid or branched fatty acids, such as. B. isostearic acid.
Aqueous alkyldiketene dispersions usually contain 10-20 wt.% AKD, as well as protective colloids active as retention aids , especially polycations, such as. B. cationic starch , copolymers of N- vinylpyrrolidone and quaternized 1-vinylimidazole , acylated polyethyleneimines or cationic high molecular weight ( mean molar mass up to 7 million g / mol) polyacrylamides (C-PAM) and other stabilizers , mostly anionic surfactants , such as. B. Ligninsulfonate or condensation products from naphthalenesulfonic acid sodium salt and formaldehyde .
AKD dispersions stabilized in this way are active and stable for up to three months at room temperature and can also tolerate the addition of different fillers for paper, cardboard or cardboard, such as B. kaolin , chalk , talc , titanium dioxide , calcium sulfate , aluminum oxide , etc. in proportions by weight of 5 to 25%.
The amounts of alkyl ketene dimer used for sizing paper and paper products are preferably 0.15 to 0.8% by weight, sometimes also 0.05 to 0.2% by weight, based on the dry paper stock.
Paper sizing with alkylated ketene dimers
A three-stage process was proposed for paper sizing with AKD, which, despite controversial discussions in the 1990s, seems to best reflect the processes taking place and explain the results achieved. Decisive criteria for the quality of the water repellency of papers are
1. the retention , d. H. the retention of the AKD particles on the moist paper pulp on the paper screen
2. the spreading , d. H. the spread of the AKD particles on the surface and the penetration of the paper pulp
3. the chemical reaction of the hydroxyl groups of the cellulose ( esterification ) with the alkylated ketene dimers with the formation of beta-ketocarboxylic acid esters.
Ad 1. The decisive factor here is the molecular structure, i. H. molar mass and degree of cross-linking, the chemical nature, d. H. the molar charge density of cationic groups, the exact dosage of the cationic polymer as dispersion stabilizer and retention aid, as well as compliance with the other process parameters such as temperature, pH and residence times.
Ad 2. After removing the excess water - also to avoid hydrolysis of the AKD to beta-keto acid and subsequent decarboxylation to the ketone -
The stabilized AKD particles are broken up on the base paper mass, the solid AKD wax is melted (at approx. 90 ° C), the liquid AKD wax is spread by surface diffusion on the cellulose fibers and the formation of hydrophobic layers that are as closed as possible, their thickness depends on the AKD concentration in the dispersion.
Ad 3. The hydrophobization of cellulose fibers with alkylated ketene dimers takes place most effectively in a neutral, preferably weakly alkaline (pH 7.5-9.0) medium. The reaction temperature is usually 90–110 ° C, with around 40% of the AKD used reacting with the cellulose. The contact angles of> 100 ° measured after the reaction indicate the hydrophobic character of the AKD-modified model surfaces. The esterification of hydroxyl groups on cellulose fibers could also be demonstrated by comparison reactions with 14 C-labeled AKD.
Sizing with AKD is suitable for the permanent waterproofing of newsprint, printing and writing papers and of cardboard boxes as containers for liquids (also for food, such as milk), as well as for improving the dimensional stability of paper products and the runnability of Paper machines.
literature
- JC Roberts: Paper Chemistry, 2nd Edition . Ed .: JC Roberts. Chapman & Hall, London 1996, ISBN 978-0-7514-0236-0 .
- D. Johnson: Applications of Wet-End Paper Chemistry, 2nd Edition . Ed .: I. Thorn, CO Au. Springer Netherlands, 2009, ISBN 978-1-4020-6037-3 , pp. 73-112 .
Individual evidence
- ^ E. Wedekind: About the production of acid chlorides with the help of tertiary amines . In: Ber. German Chem. Ges. Volume 34 , no. 2 , 1901, p. 2070-2077 , doi : 10.1002 / cber.19013402122 .
- ^ E. Wedekind: On the behavior of some acid chlorides in the removal of hydrogen chloride . In: Liebigs Ann. Chem. Band 323 , no. 2 , 1902, pp. 246-257 , doi : 10.1002 / jlac.19023230206 .
- ↑ a b E. Wedekind, W. Weisswange: About the synthesis of a diketone of the cyclobutane series . In: Ber. German Chem. Ges. Volume 39 , no. 2 , 1906, p. 1631-1646 , doi : 10.1002 / cber.19060390287 .
- ↑ E. Wedekind, J. Haeussermann: Pyrononsynthesen using the tertiary base reaction I . In: Ber. German Chem. Ges. Volume 41 , no. 2 , 1908, p. 2297-2302 , doi : 10.1002 / cber.190804102135 .
- ↑ E. Wedekind, J. Häussermann, W. Weisswange, M. Müller: Pyronone syntheses with the help of the tertiary base reaction II . In: Liebigs Ann. Chem. Band 378 , no. 3 , 1911, pp. 261-292 , doi : 10.1002 / jlac.19113780302 .
- ^ H. Staudinger: Ketene, a new body class . In: Ber. German Chem. Ges. Volume 38 , no. 2 , 1905, p. 1735-1739 , doi : 10.1002 / cber.19050380283 .
- ^ H. Staudinger, HW Klever: About Ketene. 5th communication. Reactions of dimethyl ketene . In: Ber. German Chem. Ges. Volume 40 , no. 1 , 1907, p. 1149-1153 , doi : 10.1002 / cber.190704001170 .
- ↑ NTM Wilsmore, AW Stewart: ketene. Comments on the treatise by HHrn. Staudinger and Klever . In: Ber. German Chem. Ges. Volume 41 , no. 1 , 1908, p. 1025-1027 , doi : 10.1002 / cber.190804101202 .
- ^ R. Huisgen, P. Otto: The mechanism of dimerization of dimethylketene . In: J. Am. Chem. Soc. tape 90 , no. 19 , 1968, p. 5342-5343 , doi : 10.1021 / ja.01021a090 .
- ↑ RH Hasek, RD Clark, GL Mayberry: DIMETHYLKETENE β-LACTONE DIMER In: Organic Syntheses . 48, 1968, p. 72, doi : 10.15227 / orgsyn.048.0072 ; Coll. Vol. 5, 1973, p. 1103 ( PDF ).
- ↑ a b Patent US2369919 : Ketoethenones and process therefor. Registered October 13, 1938 , published February 20, 1945 , applicant: EI du Pont de Nemours & Co., inventor: JC Sauer.
- ↑ JC Sauer: Ketene dimers from acid chlorides . In: J. Am. Chem. Soc. tape 69 , no. 10 , 1947, pp. 2444–2448 , doi : 10.1021 / ja.01202a058 .
- ↑ Z. Zhang, G. Li, G. Hu, Y. Sun: Theoretical research on the mechanism of the dimerization reaction of alkyl ketenes . In: J. Chem. Band 2013 , 2013, doi : 10.1155 / 2013/481586 .
- ↑ Patent US5484952 : Process for the manufacture of alkyl ketene dimer. Applied on May 5, 1994 , published January 16, 1996 , Applicant: Hercules Inc., Inventors: TF Nolan, BM Stubbs.
- ↑ Patent US7960497B2 : Preparation of alkyl ketene dimers. Registered on January 3, 2007 , published June 14, 2011 , applicant: Hercules Inc., inventor: DA Gerstenhaber.
- ↑ Patent US5344943 : Long-chain ketene dimers. Applied on December 29, 1992 , published on September 6, 1994 , applicant: Akzo Nobel BV, inventor: N. Brolund.
- ↑ a b Patent WO03045936A1 : Method for producing alkyl ketene dimers. Registered on November 19, 2002 , published on June 5, 2003 , applicant: BASF AG, inventor: R. Ettl, M. Winter, T. Freund, T. Kessler, G. Grimm.
- ↑ Patent US2627477 : Higher alkyl ketene dimer emulsion. Filed October 6, 1949 , published February 3, 1953 , applicant: Hercules Powder Co., inventor: WF Downey.
- ↑ Patent WO9626318 : Aqueous alkyldiketene dispersions and the use thereof as glue for paper. Applied on February 7, 1996 , published on August 29, 1996 , Applicant: BASF AG, Inventors: R. Ettl, P. Lorencak, G. Scherr, W. Reuther, G. Glas.
- ↑ Patent WO2007141197A1 : Aqueous alkylketene dimer dispersions. Registered on June 1, 2007 , published on December 13, 2007 , applicant: BASF AG, inventor: C. Hamers, A. Brockmeyer, M. Schmid, K. Lorenz, U. Riebeling.
- ↑ a b c T. Lindström, T. Larsson: A note on AKD-sizing: an investigation of real and apparent contradictions in literature regarding spreadin / diffusion of AKD on cellulose, Report no.81 . Ed .: STFI-Packfors. 2005 ( online [PDF]).
- ↑ J. Lindfors, J. Sahmi, J. Laine, P. Stenius: AKD and ASA model surfaces: preparation and characterization . In: BioResources . tape 2 , no. 4 , 2007, p. 652-670 ( PDF; 938 KB ).