Allyl thiourea
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
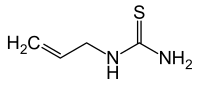
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Allyl thiourea | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 8 N 2 S | ||||||||||||||||||
| Brief description |
white, unpleasant smelling solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 116.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.22 g cm −3 (25 ° C) |
||||||||||||||||||
| Melting point |
70-72 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| Refractive index |
1.5936 (78 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Allylthiourea is a urea - derivative selected from allyl isothiocyanate and ammonia can be manufactured. It was first synthesized by Dumas and Pelouze at the end of the 19th century through the action of ammonia on mustard oil .
properties
Allylthiourea is a white, unpleasant (somewhat like garlic) smelling, bitter-tasting, flammable solid. It is moderately soluble in water but soluble in ethanol . The compound has a monoclinic crystal structure with the space group P 2 1 / a (space group no. 14, position 3) with four molecules in a cell with the dimensions a = 9.819, b = 8.553, c = 9.170 Å , β = 127, 3 ° and Z = 4.
use
When analyzing wastewater , allyl thiourea is used as a nitrification inhibitor . The microorganisms are inhibited so that the oxygen present is not used for nitrate production, but is available for the oxidation of carbon to carbon dioxide . Such substances are generally undesirable in biological wastewater treatment. When analyzing (e.g. BOD analysis ) the wastewater, they can be added in order to prevent additional consumption of oxygen. The oxygen consumption for nitrification can be estimated from an analysis with and an analysis without the addition of allyl thiourea.
Allyl thiourea is used in analog photography as a solvent for silver halides and to increase sensitivity through chemical sensitization. Since, according to older studies, it has a scar-softening effect when applied locally to scar tissue, it was used accordingly. Because of the uncertain evidence of this effect, it is currently no longer used.
Individual evidence
- ↑ a b c d e f Entry on allyl thiourea in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c Data sheet N-Allylthiourea (PDF) from Merck , accessed on March 20, 2011.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-12.
- ↑ Entry on allyl thiourea. In: Römpp Online . Georg Thieme Verlag, accessed on November 12, 2014.
- ↑ E. Schmidt, J. Gadamer: About thiosinamine and its halogen addition products. In: Archiv der Pharmazie , 1895, 233 (9), 646–684. doi : 10.1002 / ardp.18952330903 .
- ^ A b c Franz von Bruchhausen: Hager's Handbook of Pharmaceutical Practice , Volume 4, p. 52.
- ^ The Crystal and Molecular Structure of Thiosinamine ( Memento June 26, 2006 in the Internet Archive ).
- ↑ Allylthiourea (water knowledge) .
- ↑ Thiosinamine (Fotolabor.de) .
