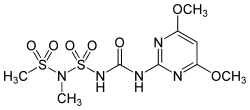
Amidosulfuron
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Amidosulfuron | |||||||||||||||
| other names |
1- (4,6-Dimethoxypyrimidin-2-yl) -3- ( N -mesyl- N -methylsulfamoyl) urea |
|||||||||||||||
| Molecular formula | C 9 H 15 N 5 O 7 S 2 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 369.37 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.51 g cm −3 (bulk density) |
|||||||||||||||
| Melting point |
179 ° C |
|||||||||||||||
| Vapor pressure |
<0.001 hPa (25 ° C) |
|||||||||||||||
| pK s value |
3.58 |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Amidosulfuron is a chemical compound from the group of sulfonylureas and pyrimidines .
Extraction and presentation
Amidosulfuron can be obtained by reacting methane- N -methylsulfonamide with chlorosulfonyl isocyanate and 2-amino-4,6-dimethoxypurimidine .
properties
Amidosulfuron is a colorless solid with a slightly acidic odor, which is soluble in water, whereby the water solubility is strongly dependent on the pH value . At 20 ° C this is 9 mg l −1 at a pH value of 5.8, and 13,500 mg l −1 at a pH value of 10.0. The compound is stable to photolytic degradation.
use
Amidosulfuron is preferred as a selective post-emergence herbicide against broad-leaved weeds. The effect is based on the inhibition of the amino acid synthesis of valine and isoleucine by inhibiting the acetolactate synthesis . The connection is mainly made through the leaves.
Admission
Amidosulfuron has been approved in Germany since 1994.
In Germany, Austria and Switzerland, plant protection products (e.g. Hoestar) with this active ingredient are approved.
Individual evidence
- ↑ a b c d e f Amidosulfuron data sheet at Sigma-Aldrich , accessed on May 20, 2017 ( PDF ).
- ↑ a b c d e Entry on Amidosulfuron in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on August 1, 2013.
- ↑ Entry on amidosulfuron in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on Amidosulfuron (ISO) in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on March 12, 2017. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 165 ( limited preview in Google Book search).
- ↑ a b M. Bahadir, H. Parlar, Michael Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 978-3-540-63561-1 , pp. 80 ( limited preview in Google Book search).
- ↑ Peter Brandt (Ed.): Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products ; Approval history and regulations of the Plant Protection Application Ordinance . Springer, 2010, ISBN 978-3-0348-0028-0 , pp. 8 ( limited preview in Google Book search).
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on amidosulfuron in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 13, 2016.
