Aminomethylphosphonic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
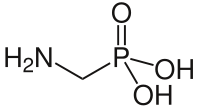
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Aminomethylphosphonic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CH 6 NO 3 P | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 111.04 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
286.5 ° C (dec.) |
||||||||||||||||||
| solubility |
soluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Aminomethylphosphonic acid (also aminomethanephosphonic acid , aminomethylphosphonic acid , AMPA) is the main breakdown product of the broad spectrum herbicide glyphosate . AMPA is mineralized by the soil microflora, but at a lower rate than glyphosate itself. AMPA was found in the environment more frequently and in higher concentrations than glyphosate.
The AMPA metabolite is also formed as a breakdown product of nitrogen-containing organic phosphonates (aminopolyphosphonates) such as ATMP, EDTMP and DTPMP. Since phosphonates are used in large quantities in detergents , as inhibitors against corrosion and scale formation in cooling and boiler feed water, and in the textile and paper industry, it is not easy to determine which source this is due to the detection of AMPA in water.
properties
AMPA is an analogue of the amino acid glycine , with the COOH group being replaced by a PO (OH) 2 group (phosphonic acid group). In contrast to the amino (mono) carboxylic acids, phosphonic acids are dibasic; d. H. they can donate two protons to bases, e.g. B. on H 2 O molecules when they are dissolved in water. However, since AMPA contains an amino group H 2 N–, which is a stronger base than H 2 O, the amino group captures 1 proton and thus becomes a positively charged ammonio group (H 3 N + -). This creates a zwitterion , which should already be present in the solid state. This is indicated by the relatively high melting or decomposition point.
In principle, the PO (OH) 2 group of AMPA can donate two protons; H. dissociate completely. The acidity of the zwitterion is therefore described by three pK a values . The pK a1 was found to be 1.8; pK a2 = 5.4 and pK a3 = 10.0. The isoelectric point is at pH 3.45.
synthesis
Syntheses of the compound were reported before it was recognized as a metabolite of glyphosate. Production from simple educts was difficult; protection groups had to be used. For example, Kabachnik and Melved synthesized the compound from diethyl phosphonate ( diethyl phosphite ), ammonia and formaldehyde (the reaction is to be regarded as a special case of the later Kabachnik-Fields reaction ). The aminomethanephosphonic acid ester formed was then hydrolyzed. The reaction of phosphonic acid, formaldehyde and ammonia, which is often used for the production of alpha-aminomethylphosphonic acids, did not achieve the goal in this case.
Web links
- Joint Meeting on Pesticide Residues (JMPR), Monograph for Aminomethylphosphonic Acid (AMPA)
Individual evidence
- ↑ James R. Chambers and AF Isbell. The Journal of Organic Chemistry, 29: 832-836 (1964).
- ↑ a b c data sheet (aminomethyl) phosphonic acid, 99% from Sigma-Aldrich , accessed on October 21, 2011 ( PDF ).
- ↑ J. Schuette: Environmental Fate of Glyphosate , 1998, Department of Pesticide Regulation, Sacramento ( PDF ).
- ^ EA Scribner, WA Battaglin, RJ Gilliom, MT Meyer: Concentrations of Glyphosate, Its Degradation Product, Aminomethylphosphonic Acid, and Glufosinate in Ground- and Surface-Water, Rainfall, and Soil Samples Collected in the United States, 2001-06 , United States Geological Survey Scientific Investigations Report 2007-5122 ( PDF ).
- ↑ Printed matter 17/7168 of the German Bundestag, answer of the federal government to the small question "Risk assessment and approval of the herbicidal active ingredient glyphosate" (Question 19) , September 27, 2011.
- ↑ a b Chen, Zuliang; He, Wenxiang; Beer, Michael; Megharaj, Mallavaranpu; Naidu, Ravendra: Speciation of glyphosate, phosphate and aminomethylphosphonic acid in soil extracts by ion chromatography with inductively coupled plasma mass spectrometry with an octopole reaction system. Talanta Vol. 78 (2009), pp.852-856, Issue 3, cited from doi: 10.1016 / j.talanta.2008.12.052 .
- ↑ James R. Chambers and AF Isbell, The Journal of Organic Chemistry, Vol. 29 (1964), pp. 832-836.
- ↑ Martin I. Kabachnik, T. Ya. Melved: Новый метод синтеза сс-аминофосфиновых кислот (A new method for the synthesis of α-amino phosphonic acids ) . In: Doklady Akademii Nauk SSSR . tape 83 , 1952, pp. 689 .
- ↑ Kurt Moedritzer and Riyad R. Irani: The Direct Synthesis of alpha-Aminomethylphosphonic Acids. Mannich-Type Reactions with Orthophosphorous Acid. The Journal of Organic Chemistry, Vol. 31, 1966, pp. 1603-1607.


