Ammonium dichromate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
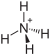 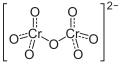
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ammonium dichromate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | (NH 4 ) 2 Cr 2 O 7 | ||||||||||||||||||
| Brief description |
orange-colored, odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 252.07 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.15 g cm −3 |
||||||||||||||||||
| Melting point |
170 ° C |
||||||||||||||||||
| solubility |
good in water (360 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Authorization procedure under REACH |
of particular concern : carcinogenic, mutagenic, toxic for reproduction ( CMR ); subject to approval |
||||||||||||||||||
| MAK |
Switzerland: 5 μg m −3 (calculated as chromium) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ammonium dichromate is an ammonium salt of dichromic acid . It has the formula (NH 4 ) 2 Cr 2 O 7 .
properties
Ammonium dichromate is an orange powder that is readily soluble in water.
Due to its composition of an easily oxidizable cation (ammonium ion) and a strong oxidizing agent ( dichromate ), it can decompose exothermically.
The best known experiment for the decomposition of crystalline ammonium dichromate is the volcano experiment. A larger crystal or a small pile is ignited on top. After ignition, the reaction proceeds with vigorous annealing, noise ( nitrogen evolution) and the formation of loose gray-green dichromium continued. The dichromium trioxide formed oozes out of the reaction site like volcanic ash and thus forms a cone. The corresponding equation for this reaction is:
The volcano test is prohibited in German schools due to the formation of chromium (III) chromate .
Overall, ammonium dichromate is a strong oxidizing agent with strong reducing agents such as finely divided metal powders, sulfur or phosphorus reacts very violently (explosive).
Because of its ionic nature and the dissociation equilibria formed in water, ammonium dichromate has a corrosive effect.
Explosive properties
Ammonium dichromate decomposes exothermically above 100 ° C ; between 130 and 180 ° C self-ignition occurs; the reaction is explosive even in the absence of oxygen from 240 ° C. In the case of an initial ignition using picric acid, it detonates only incompletely, even when dammed.
The sensitivity to mechanical stress is extremely low. Ammonium dichromate does not react at all when rubbed in a non-glazed mortar; the sensitivity to impact corresponds roughly to that of ammonium perchlorate (15 cm under a 10 kg drop hammer; ammonium nitrate , which is not classified as explosive, detonates under the 10 kg drop hammer from a height of 20 cm). The detonation only propagates over very short distances, even under optimal conditions.
Ammonium dichromate is not an explosive , but is used occasionally in pyrotechnic charges and as a catalyst in propellants based on ammonium nitrate.
Toxicology and Ecotoxicology
Ammonium dichromate is toxicologically classified by the EU Commission as:
Carcinogenic Category: 1B (H350: May cause cancer.)
Mutagen category: 1B (H340: May cause genetic defects.)
Toxic to reproduction Category: 1B (H360FD: May damage fertility. May damage the unborn child.)
It is also classified as life-threatening if inhaled (H330) and toxic if swallowed (H301). Contact with the skin is rated as harmful (H312). Another danger from ammonium dichromate is the possibility of sensitization through inhalation and skin contact (H334 / 317).
Ecotoxicologically, it is considered to be very toxic to aquatic organisms and can have a long-term harmful effect on water (H410). In the administrative regulation for water-endangering substances (VwVwS as of July 2005), ammonium dichromate with identification number 290 is classified in the highest water hazard class 3. Due to the bioconcentration factor of 200–2000 given in the literature, accumulation in organisms is possible.
use
- In the analog photography , holography and the precious printing process (especially in the light printing and flexographic printing as well as (together with gelatin, polyvinyl alcohol and, if necessary, dyes) the so-called "dichromated gelatin" (DCG) holograms):
ammonium dichromate tans exposure to light gelatin or other colloids , whereby these sensitive to light and, after exposure and development, become a color layer or a color carrier or hologram. - In pyrotechnic articles.
- For the production of wood preservatives for industrial use.
- For the production of chromium (IV) oxide for magnetic data carriers.
- For the production of catalysts for organic synthesis .
Special legal regulations
Ammonium dichromate is subject to the Chemicals Prohibition Ordinance , the Water Management Act and the Explosives Act . In industrial quantities, it is also subject to the Hazardous Incident Ordinance .
There are also employment restrictions for young people ( Youth Labor Protection Act ) , as well as for expectant and nursing mothers ( Maternity Protection Directive ).
Individual evidence
- ↑ a b c d data sheet ammonium dichromate at AlfaAesar, accessed on January 14, 2020 ( PDF )(JavaScript required) .
- ↑ a b c Entry on ammonium dichromate in the GESTIS substance database of the IFA , accessed on January 14, 2020(JavaScript required) .
- ↑ Entry on Ammonium dichromate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry in the SVHC list of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ Entry in the register of substances subject to authorization of the European Chemicals Agency , accessed on July 14, 2014.
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for chromium (VI) compounds ), accessed on October 27, 2015.
- ↑ Entry on ammonium dichromate. In: Römpp Online . Georg Thieme Verlag, accessed on December 9, 2015.
- ↑ M. Binnewies et alii: Allgemeine und Anorganische Chemie . 2nd Edition. Spectrum, 2010, ISBN 3-8274-2533-6 , pp. 676 .
- ↑ Classes in schools with hazardous substances (online version; PDF; 10.0 MB), p. 21, footnote 2, accessed on April 25, 2011. However, according to Gmelin, only chromium (III) oxide, nitrogen and water vapor are produced. Even after more recent scientific studies (D. DeWaal et al., Journal of Solid State Chemistry 80, 170 (1989)), no formation of the feared chromium (III) chromate (which is subject to restrictions on use as a CMR substance in chemical law) could be detected.
- ^ Tadeusz Urbanski: Chemistry and Technology of Explosives. Vol. II, Pergamon Press, 1965, p. 490.
- ↑ H. Kast; In: Z. ges. Shooting explosives. 22 [1927] 6/9: “Under the heat of the detonation of 30 g of pressed picric acid on the salt stuffed into a 4 cm wide, 4 mm thick and 18 cm long tube (presumably Zn tube), disintegration occurs only along a short distance the verb. "
- ^ Leopold Gmelin: Gmelin's handbook of inorganic chemistry. 8th edition. System no. 52, p. 714.
- ^ Josef Köhler: Explosives. Wiley-VCH, 2008, p. 17.
- ↑ K. Kurokawa et al., Applied Optics 37, 3038 (1998).
- ↑ KUBOTA HOLOGRAPHY LAB: Examples of DCG holograms , accessed June 3, 2013.

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)






