Androstenediol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Androstenediol | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 19 H 30 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 290.44 g mol −1 | |||||||||||||||||||||
| Melting point |
178-182 ° C |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
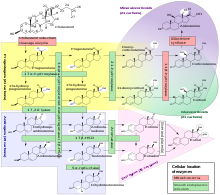
Androstenediol , more specifically 5-androsten-3 β , 17 β -diol or Hermaphrodiol, is a biologically active steroid. As a steroid hormone , it is a chemical intermediate stage in the biosynthesis of testosterone from pregnenolone via dehydroepiandrosterone (DHEA).
Biological importance
Two synthetic pathways are discussed in the testosterone biosynthesis from pregnenolone, the Δ 4 metabolic pathway via (progesterone-hydroxprogesterone-androstenedione) and the Δ 5 metabolic pathway via (hydroxypregnenolone-dehydroepiandrosterone-androstenediol). These processes take place in the Leydig cells of the testes. When androstenediol is converted to testosterone, the hydroxyl group at C-3 is oxidized to the keto group.
The steroid hormones can be converted into one another by enzymes (dehydrogenase or isomerase or the P450-dependent enzymes or aromatase). It is characteristic of the male ( androgenic ) sex hormone testosterone that the A-ring of the steroid structure ( sterane ) is not aromatic. It can be aromatized under the action of the enzyme aromatase , which in turn is produced in the endoplasmic reticulum (ER) of the renal cortex, and thus converted into an estrogen .
Androstenediol binds to an estradiol receptor during biosynthesis. The binding affinity of steroids to their receptors is primarily regulated by the presence of keto and hydroxyl groups, so oxidoreductases and 17 β -hydroxysteroid dehydrogenases play a role in steroid modulation. 5-Androstenediol is a direct metabolite of the steroid dehydroepiandrosterone (DHEA), which is produced in the human cerebral cortex. It is less androgenic than 4-androstenediol and has been shown to affect the immune system. Androstenediol has an estrogenic effect, similar to DHEA. Because it induces the production of white blood cells and blood plasma , it can be used as an indicator of ionizing radiation (Neumune HE2100). This intermediate stage is also of importance for the development of drugs against prostate cancer . Since not only healthy prostate tissue, but also prostate cancer cells are androgen-dependent, this cancer can be treated with hormonal blockade or antiandrogens . It also affects hair growth.
properties
It is a solid. Androstenediol is chiral and has 7 stereocenters.
literature
- H. Lüllmann, K. Mohr, M. Wehling, L. Hein: Pharmacology and Toxicology. 18th edition, Thieme Verlag 2016, pp. 476–478. ISBN 978-3-13-368518-4 .
- Teresa Kołek et al .: Hydroxylation of DHEA, androstenediol and epiandrosterone by Mortierella isabellina AM212. Evidence indicating that both constitutive and inducible hydroxylases catalyze 7α- as well as 7β-hydroxylations of 5-ene substrates. In: Org. Biomol. Chem. 2011, 9, p. 5414. doi: 10.1039 / c1ob05350g .
- Cherkasov Artem et al .: An Updated Steroid Benchmark Set and Its Application in the Discovery of Novel Nanomolar Ligands of Sex Hormone-Binding Globulin. In: Journal of Medicinal Chemistry . 2008, doi: 10.1021 / jm7011485 .
Individual evidence
- ↑ Entry on 5-Androstenediol at ChemicalBook , accessed June 24, 2017.
- ↑ a b Data sheet 5-Androstene-3β, 17β-diol from Sigma-Aldrich , accessed on August 26, 2017 ( PDF ).
- ^ Mikael Häggström, David Richfield: Diagram of the pathways of human steroidogenesis. In: WikiJournal of Medicine. Volume 1, 2014, doi: 10.15347 / wjm / 2014.005 .
- ↑ G. Löffler, P. Petrides: Biochemistry and Pathobiochemistry. Springer Verlag, 2003, 7th edition, ISBN 3-540-42295-1 , p. 891.
- ↑ D. Ganten, K. Ruckpul: Molecular medical principles of paracrine and autocrine regulation disorders . Springer Verlag, 2006, ISBN 978-3-540-28781-0 , pp. 547-549.
- ^ DS Coffey: The Physiology of Reproduction . Ed .: E. Knobil, J. Neill. Raven Press, New York 1988, ISBN 978-0-88167-281-7 , chapter Androgen action and the sex accessory tissues , pp. 1081-1119 .
- ↑ Reinhard Hackenberg, Inga Turgetto, Angelika Filmer, Klaus-Dieter Schulz: Estrogen and androgen receptor mediated stimulation and inhibition of proliferation by androst-5-ene-3β, 17β-diol in human mammary cancer cells . In: The Journal of Steroid Biochemistry and Molecular Biology . 46, No. 5, 1993, pp. 597-603. doi : 10.1016 / 0960-0760 (93) 90187-2 .
- ↑ George GJM Kuiper, Bo Carlsson, Kaj Grandien, Eva Enmark, Johan Häggblad, Stefan Nilsson, Jan-Åke Gustafsson: Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta . In: Endocrinology . tape 138 , no. 3 , March 1997, p. 863-870 , doi : 10.1210 / endo.138.3.4979 , PMID 9048584 .
- ^ Rob Bradbury: Cancer . Springer Science & Business Media, 2007, ISBN 978-3-540-33120-9 , p. 43.
- ^ MH Whitnall, TB Elliott, RA Harding, CE Inal, MR Landauer, CL Wilhelmsen, L. McKinney, VL Miner, WE Jackson 3rd, RM Loria, GD Ledney, TM: Androstenediol stimulates myelopoiesis and enhances resistance to infection in gamma-irradiated mice. In: International Journal of Immunopharmacology . Volume 22, Number 1, January 2000, pp. 1-14, PMID 10684984 , doi: 10.1016 / s0192-0561 (99) 00059-4 .
- ↑ Z. Culig, J. Stober, A. Gast, H. Peterziel, A. Hobisch, C. Radmayr, A. Hittmair, G. Bartsch, AC Cato, H. Klocker: Activation of two mutant androgen receptors from human prostatic carcinoma by adrenal androgens and metabolic derivatives of testosterone. In: Cancer detection and prevention . Volume 20, Number 1, 1996, pp. 68-75, PMID 8907206 .
- ↑ F. Horn, G. Lindenmeier et al .: Human Biochemistry. Thieme Verlag 2002, p. 405, ISBN 3-13-130882-6 .