Boron carbide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
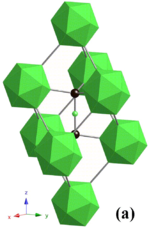
| Crystal structure of B 4 C. Green: B 12 icosahedron and boron atoms. Black: carbon atoms | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Boron carbide | ||||||||||||||||||
| other names |
Black Diamond |
||||||||||||||||||
| Ratio formula | B 4 C | ||||||||||||||||||
| Brief description |
shiny black crystals or black powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 55.25 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.51 g cm −3 |
||||||||||||||||||
| Melting point |
2350 ° C |
||||||||||||||||||
| boiling point |
> 3500 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Boron carbide (empirical formula B 4 C ) is a very hard material with a Mohs hardness of 9.3, which is used as a wear-resistant material. Boron carbide is often classified as a metal carbide, but this is very controversial because boron has no metallic properties. The difference in electronegativity between boron and carbon is significantly less than 1.5; in contrast to metallic (intercalation) carbides such as titanium carbide (TiC), covalent non- metal- non-metal bonds are present.
Extraction and presentation
Depending on the manufacturing process, boron carbide is obtained as a fine black powder or in the form of coarse, shiny black crystals .
The coarsely crystalline boron carbide is produced from boron trioxide and carbon in an electric resistance furnace at 2400 ° C:
- Production of coarse crystalline boron carbide
The finely crystalline powder produced during the reduction of boron trioxide with magnesium in the presence of carbon :
- Manufacture of powdered boron carbide
properties
Physical Properties
Boron carbide is a non-oxide ceramic , which, like silicon carbide or silicon nitride, is characterized by its particular hardness and toughness. Boron carbide ceramic is very wear-resistant at low temperatures. In terms of its hardness, it is more wear-resistant than silicon nitride ceramics at low temperatures (see below).
Boron carbide is an effective neutron absorber and was z. B. thrown into the exploded reactor during the Chernobyl disaster in order to contain the chain reaction.
Wear mechanism
Boron carbide, like other materials, wears mechanically in that parts of the structure are broken off by the action of force (impact, friction). But since boron carbide is a very hard material, it is only subject to this wear to a small extent. Silicon nitride and silicon carbide are similarly hard and wear-resistant.
Boron carbide resists oxidation through passivation by causing the surface of the material to react with oxygen :
-
or.
During the reaction, the temperature is essential, as a result of the Boudouard equilibrium , carbon monoxide or carbon dioxide can be formed , depending on the case . The boron trioxide formed is a solid that melts in crystalline form at 450 ° C, in amorphous or glassy form at a lower temperature. Its melt prevents oxygen from entering the boron carbide matrix, so that further oxidation is hindered. However, temperatures well above 450 ° C are often reached in wear applications, at which boron trioxide has very low viscosities . Then atmospheric oxygen can more easily diffuse through the boron trioxide melt . In the oxidation of the competing materials silicon nitride and silicon carbide , on the other hand, silicon dioxide is formed, which is much more viscous than boron trioxide and therefore less diffuses the oxygen in the air through its melt. Therefore, silicon nitride or silicon carbide is more resistant to oxidation.
Chemical properties
Boron carbide is a chemically very inert material. It is only noticeably attacked by chlorine or oxygen above 1000 ° C. Against hydrogen fluoride and hot nitric acid it is completely resistant.
use
- Armor material
- Neutron absorbers in nuclear power plants
- Material for sandblasting nozzles
Individual evidence
- ↑ a b Entry on bar carbide. In: Römpp Online . Georg Thieme Verlag, accessed on December 22, 2014.
- ↑ a b c data sheet boron carbide from AlfaAesar, accessed on February 9, 2010 ( PDF )(JavaScript required) .
- ↑ a b data sheet boron carbide from Sigma-Aldrich , accessed on March 14, 2011 ( PDF ).






