Budesonide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Basic structural formula (stereocenter in position 22 is marked with a * ) | |||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Budesonide | ||||||||||||||||||
| other names |
16 α , 17 - [( RS ) -butane-1,1-diyldioxy] -11 β , 21-dihydroxypregna-1,4-diene-3,20-dione ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 25 H 34 O 6 | ||||||||||||||||||
| Brief description |
white to almost white, crystalline powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Drug class | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 430.53 g · mol -1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
226 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Budesonide is a synthetic glucocorticoid and is used as a drug for the topical treatment of bronchial asthma , COPD , non- infectious rhinitis (such as hay fever ), nasal polyps , inflammatory bowel disease (such as Crohn's disease or ulcerative colitis ) and chronic inflammation of the liver due to autoimmune hepatitis .
Stereoisomerism
Budesonide is a mixture of diastereomers in which the configuration of all stereocenters is identical, with one exception at C-22; therefore the two diastereomers with the (22 R ) and (22 S ) configuration are in a ratio of 1: 1.
The (22 R ) isomer is also known as dexbudesonide .
| Isomers of budesonide | ||
| Surname | (22 R ) -Budesonide | (22 S ) -Budesonide |
| other names | Dexbudesonide | |
| Structural formula |

|
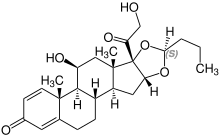
|
| CAS number | 51372-29-3 | 51372-28-2 |
| 51333-22-3 (mixture) | ||
| EC number | 257-161-7 | 257-160-1 |
| 257-139-7 (mixture) | ||
| ECHA info card | 100.051.947 | 100.051.946 |
| 100.007.162 (mixture) | ||
| PubChem | 40000 | 63006 |
| 5281004 (mixture) | ||
| Wikidata | Q27254753 | Q27251792 |
| Q422212 (mixture) | ||
effect
Budesonide works where it is supplied or released (as a drop or spray in the nose, as an inhalation in the bronchial system, as capsules or enema in the intestine). If the substance gets into the bloodstream - for example through ingestion and absorption - its effectiveness on the entire organism (“systemic effect”) is only slight due to the strong breakdown during the first passage through the liver ( first-pass effect ). Therefore, the typical side effects of glucocorticoids are less pronounced.
If budesonide is inhaled, a fungal infection in the oral cavity or hoarseness may occur. Therefore, this application should always take place before a meal or the mouth should be rinsed well afterwards. In the case of inhalation, it should also be noted that the active ingredient takes 1–4 weeks to take effect, as it is intended for long-term treatment.
Budesonide can also be used in the acute episode of Crohn's disease when the ascending part of the colon is affected. The therapy is less effective than with other glucocorticoids, but there are fewer undesirable effects. There are also preparations for the treatment of ulcerative colitis. In particular if the inflammation is limited to the rectum, local treatment with enema is relatively effective and at the same time has few side effects. Here, too, the full effect only sets in after 2–4 weeks.
Side effects
Common side effects when taking budesonide are:
- hoarseness
- to cough
- Irritations in the mouth and throat
Contraindication / Contraindications
Caution should be exercised when taking birth control pills , as the hormone ethinylestradiol is broken down by the same enzyme as the active ingredient in asthma. This can increase the concentration of budesonide in the blood and make it more effective.
In the case of milk protein sensitivity, the active ingredient must not be used.
Pregnant and breastfeeding women should consult a doctor before use and only use budesonide after weighing up all the risks. The active ingredient can be transferred through the placenta to the unborn child and breast milk. In a study of 6,000 pregnancies, there was no indication that the child was at risk.
Trade names
Aquacort (D), Budapp (D), Budecort (D), Budenobronch (D, A), Budenofalk (D, CH), Budes (D), Budiair (D, A), Budo-San (A), Cortiment ( D), Cortinasal (CH), Cyclocaps Budesonid (D), Entocort (D, A, CH), Entocort rectal (D), Jorveza (D), Giona (A), Miflonide (D, A, CH), Novolizer Budesonid (A), Novopulmon (D), Pulmax (D), Pulmicort (D, A, CH), Rhinocort (A, CH), various generics (D, A, CH)
Combination preparations with formoterol : Symbicort (D, A, CH), Vannair (CH)
Individual evidence
- ↑ a b c European Pharmacopoeia Commission (Ed.): EUROPÄISCHE PHARMACOPÖE 5th EDITION . tape 5.0-5.8 , 2006.
- ^ Entry on budesonide in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c Paul Beringer. Remington: the science and practice of pharmacy. Lippincott Williams & Wilkins, 2005, ISBN 0-7817-5211-6 , p. 1446 ( limited preview in Google book search).
- ↑ Budesonide data sheet at Sigma-Aldrich , accessed on December 17, 2012 ( PDF ).
- ↑ a b c d Entry on budesonide. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ^ Autoimmune Hepatitis - Yael Foundation. Retrieved July 26, 2017 .
- ↑ J. Albertsson, A. Oskarson, C. Svensson: X-ray Study of Budesonide: Molecular Structures and Solid Solutions of the (22S) and (22R) Epimers of 11β, 21-Dihydroxy-16α, 17α-propylmethylenedioxy-1, 4-pregnadiene-3,20-dione. In: Acta Crystallographica. 1978 / B34, pp. 3027-3036.
- ↑ M. Isaksson, M. Bruze, J.-P. Lepoittevin, A. Goossens: Patch Testing With Serial Dilutions of Budesonide, Its R and S Diastereomers, and Potentially Cross-Reacting Substances. In: ScienceDirect , 2000, pp. 170-176; doi : 10.1053 / ajcd.2001.20553 .
- ^ A. Otley, AH Steinhart: Budesonide for induction of remission in Crohn's disease. In: Cochrane Database Syst Rev. (4), Oct 19, 2005, Art. No. CD000296. PMID 16235274 .
- ↑ Interactions and contraindications (contraindications). Retrieved August 27, 2017 .
- ↑ Red List online, as of September 2009.
- ↑ AM comp. d. Switzerland, as of September 2009.
- ↑ AGES-PharmMed, as of September 2009.