Formoterol
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||
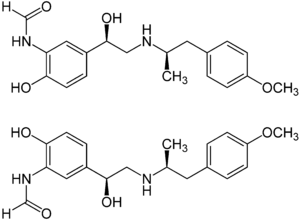
| 1: 1 mixture of ( R , R ) -formoterol (top) and ( S , S ) -formoterol (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Formoterol | |||||||||||||||||||||
| other names |
( RR , SS ) - N - (2-Hydroxy-5- {1-hydroxy-2- [1- (4-methoxyphenyl) propan-2-ylamino] ethyl} phenyl) formamide |
|||||||||||||||||||||
| Molecular formula | C 19 H 24 N 2 O 4 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 344.41 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
The formoterol is a drug selected from the group of β 2 -sympathomimetics . Administered by inhalation , it dilates the bronchi and is used to treat bronchial asthma and chronic obstructive pulmonary disease ( COPD ).
chemistry
Stereochemistry
Formoterol is a chiral drug and contains two stereocenters . Therefore there are four stereoisomers : The ( R , R ) form and the corresponding enantiomeric ( S , S ) form, as well as the ( R , S ) and ( S , R ) form. The drug formoterol consists of the racemate (1: 1 mixture) of the ( R , R ) form and the ( S , S ) form. The active isomer, the eutomer , is the ( R , R ) -enantiomer, which is also known as arformoterol and is also used therapeutically.
Dosage forms
Formoterol is available in Germany as a single substance either as a metered dose inhaler or in powder form. It is available in a fixed combination with an anti-inflammatory steroid as a powder inhaler and metered dose inhaler.
Pharmacokinetics
Only 10% of the inhaled amount reaches the bronchial mucosa. The remaining 90% are swallowed and more than two-thirds of them are quickly absorbed by the gastrointestinal tract. The maximum concentration of the active ingredient in the blood is reached after about half an hour to an hour. 61–64% of formoterol is bound to protein in the blood ( plasma protein binding ). For excretion, it is linked to a molecule of glucuronic acid directly or after splitting off a methyl group (glucuronidized) and then two thirds of it is completely eliminated with the urine and one third with the stool. On average, between 6 and 9% is excreted unchanged in the urine. The half-life is around 7 hours. It belongs to the class of long-acting beta-sympathomimetics.
application areas
Formoterol stimulates the β-receptors of the sympathetic nervous system and leads to a relaxation of the smooth muscles in the bronchi and thus to an expansion. It is therefore approved for the treatment of moderate bronchial asthma, but only in combination with an inhaled cortisone preparation. It can also be used to prevent and treat bronchial narrowing in chronic obstructive pulmonary disease and pulmonary emphysema .
Side effects
Common side effects are subtle tremors ( tremor ), headache and palpitations. Occasionally there is nausea, sweating, restlessness, dizziness, taste disorders, abnormal sensations in the mouth and throat, palpitations with cardiac arrhythmias, muscle cramps, cough, potassium deficiency in the blood as well as an increase in the blood sugar level and the concentration of insulin , free fatty acids , ketone bodies and glycerol in the blood. Rarely, angina pectoris , influencing the blood pressure in both directions, a paradoxical narrowing of the bronchi and hypersensitivity reactions with rashes, itching, hives, swelling of the face and mouth, drop in platelets ( thrombocytopenia reported) and kidney inflammation. Over-excitability with sleep disorders, hyperactive behavior and hallucinations has been observed in individual cases, especially in children under the age of twelve . Edema of the hands and feet also occurred occasionally . The use of long-acting β 2 -sympathomimetics without simultaneous administration of inhaled corticosteroids in the therapy of bronchial asthma is associated with an increased mortality.
literature
- Cates CJ, Lasserson TJ: Combination formoterol and budesonide as maintenance and reliever therapy versus inhaled steroid maintenance for chronic asthma in adults and children . In: Cochrane Database Syst Rev . No. 2, 2009, p. CD007313. doi : 10.1002 / 14651858.CD007313.pub2 . PMID 19370682 .
- Cates CJ, Lasserson TJ: Combination formoterol and inhaled steroid versus beta2-agonist as relief medication for chronic asthma in adults and children . In: Cochrane Database Syst Rev . No. 1, 2009, p. CD007085. doi : 10.1002 / 14651858.CD007085.pub2 . PMID 19160317 .
- Walters EH, Gibson PG, Lasserson TJ, Walters JA: Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid . In: Cochrane Database Syst Rev . No. 1, 2007, p. CD001385. doi : 10.1002 / 14651858.CD001385.pub2 . PMID 17253458 .
- Bartow RA, Brogden RN: Formoterol. An update of its pharmacological properties and therapeutic efficacy in the management of asthma . In: Drugs . 55, No. 2, February 1998, pp. 303-322. PMID 9506248 .
Trade names
Foradil (D, A, CH), Forair (D, A), Formatris (D), Oxis (D, A, CH), various generics (D, A)
- with fluticasone : Flutiform (D)
- with beclometasone : Foster (D, A), Inuvair (D)
- with budesonide : Symbicort (D, A, CH), Vannair (CH)
- with aclidinium bromide : Duaklir (EU), Brimica (EU)
- with beclometasone and glycopyrronium bromide : Trimbow (D)
Individual evidence
- ↑ Formoterol fumarate dihydrate data sheet from Sigma-Aldrich , accessed on May 3, 2011 ( PDF ).
- ↑ Data sheet FORMOTEROL FUMARATE DIHYDRATE CRS (PDF) at EDQM , accessed on May 27, 2008.
- ↑ Micromedex database June 23, 2012.
- ↑ Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE: Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths . In: Ann. Intern. Med. . 144, No. 12, June 2006, pp. 904-912. PMID 16754916 .
- ^ Cates CJ, Cates MJ: Regular treatment with salmeterol for chronic asthma: serious adverse events . In: Cochrane Database Syst Rev . No. 3, 2008, p. CD006363. doi : 10.1002 / 14651858.CD006363.pub2 . PMID 18646149 .
- ^ Rider, NL, Craig, TJ: A Safety Review of Long-Acting beta2-Agonists in Patients With Asthma. In: J Am Osteopath Assoc. 2006; 106 (9): pp. 562-567; PMID 17079526 .