Cockayne Syndrome
| Classification according to ICD-10 | |
|---|---|
| Q87.1 | Congenital malformation syndromes that are predominantly associated with short stature, including Cockayne syndrome |
| ICD-10 online (WHO version 2019) | |
The Cockayne syndrome (CS; also Neill-Dingwall Syndrome ) is a rare, progressive, autosomal - recessive inherited disease . It is mainly characterized by a relatively small head ( microcephaly ), little weight gain and impaired growth. Other common symptoms are delayed development that regresses as the disease progresses, hearing and vision impairment (progressive bilateral hearing loss to deafness, cataracts , retinal dystrophy , photophobia ), muscle weakness , contractures of the joints, and sensitivity of the skin to light ( photosensitivity ). The first symptoms are rarely present prenatally or shortly after birth, but in most affected individuals they do not appear until after the first year of life or later. In most cases, this makes an early diagnosis difficult. In general, there is a high clinical variability between the individual affected.
Origin of name
The Cockayne syndrome is named after the English doctor Edward Alfred Cockayne (1880-1956), who in 1936 described a pair of siblings who had the characteristics typical for CS. Ten years later, Cockayne published a second article on the siblings, in which he documented their development and gave more detailed information on family history and birth weight. In 1950, Catherine A. Neill and Mary M. Dingwall reported that two brothers had symptoms comparable to those of the siblings described by Cockayne. In their in-depth article, they compared the condition to Hutchinson-Gilford syndrome (progeria) and Werner syndrome based on the discovery of calcium deposits in people's brains and other signs of premature aging .
root cause
Cockayne syndrome, along with xeroderma pigmentosum (XP) and trichothiodystrophy (TTD), is a nucleotide excision repair disease ( DNA repair ). Affected people usually have a mutation in the CSA gene ( ERCC8 gene on chromosome 5 , gene locus q12.1) or CSB gene ( ERCC6 gene on chromosome 10, gene locus q11). In very rare cases there is a combination of a CS mutation and an XP mutation (XPB / ERCC3, XPD / ERCC2, XPG / ERCC5). (For an overview of relationships between NER factors and associated diseases, see the publication by Ferri, Orioli, & Botta, 2020.). These genes contain information for protein biosynthesis , which is involved in the repair of damaged DNA. If the DNA of cells is damaged and not repaired by appropriate proteins, the cells are restricted in their function and die. This process is part of normal human aging.
Various researchers question that a defect in the transcription-linked nucleotide excision repair (tc-NER) alone is the cause of the clinical picture, as it cannot explain all the characteristics of CS. It is believed that other CS proteins also play a role. and discusses whether mitochondrial dysfunctions and defects of the base excision repair are causally involved in the development of the clinical picture.
Characteristics and symptoms
Apart from microcephaly, the impaired weight gain and restricted growth, which occur in all those affected and which, as the disease progresses, reach a plateau on which growth levels off or stop, there are a number of other features which can, but not, occur must occur and their beginning and severity differ greatly between the individual affected.
Appearance
Children with Cockayne syndrome typically do not have any facial deformities that could aid an early diagnosis. At the beginning of their life, some children may have their eyes small and sunken ( enophthalmia ). This characteristic develops over time in all sufferers and is often associated with a progressive loss of subcutaneous and orbital adipose tissue (lipatrophy). This can also make the nose, chin and ears appear particularly prominent. Other authors describe jaw misalignments (such as retrognathia and micrognathia ), a highly curved, narrowed palate and various other dental features such as crowded teeth, hypodontia , microdontia , radiculomegaly (very long tooth roots) and enamel hypoplasia . Other characteristics described are dry skin and dry, thin or light hair.
Stunted growth and microcephaly
Restricted growth is usually the first symptom of the disease. In rare cases, growth retardation begins before birth, but usually during the first few years of life or later (see classification). Time of occurrence and degree of severity or deviation from the expected growth is usually parallel to the occurrence and degree of severity of other symptoms. After an initially rapidly flattening growth curve - compared to healthy peers - the body weight levels off to a certain value. Since the growth in height also slows down, the BMI for those less affected can be in the normal or lower normal range , despite a large deviation from those of the same age ; for those more severely affected, however, it is usually below the normal range. There may be moderate weight loss in the late stages of the disease.
Progressive microcephaly is a feature that affects all sufferers as well. At birth, the head circumference is often still within the normal range or in the lower normal range of the growth curve. In isolated cases, however, microcephaly can also be present at birth. Due to reduced growth, the head circumference of more severely affected individuals can fall below the third percentile of the growth curve for the corresponding age after some time. In those affected, on the other hand, for whom the first symptoms appear later, growth can be normal up to the age of 10.
Sensory system: hearing and seeing
Bilateral, sensorineural hearing loss is common in CS. The timing of onset varies and neurological and cognitive impairments can make diagnosis difficult. The severity of hearing loss appears to be related to the overall severity of the condition.
Cloudiness of the lens of the eye ( cataract ) occurs in around half of those affected. This can be congenital or appear later and is in most cases bilateral.
An increased sensitivity of the eyes to light ( photophobia ) also affects around half of those affected. Reduced or absent lacrimation and reduced pupils with resistance to enlargement have also been reported. A change in the pigmentation of the retina ( retinal dystrophy ) has often been described as an outstanding feature, often accompanied by a so-called "salt-and-pepper fundus". However, this only seems to become clinically relevant in the late stage and to run parallel to the overall severity of the disease.
Bones, joints and muscles
Progressive muscle and joint stiffness ( contractures ) are a common feature of people with CS. In some cases, the problems already exist after the birth, but can also appear later. The lower extremities are usually more affected than the upper ones.
A hypotonia of the trunk are also common in neurologically affected more often in combination with a muscle hypertension of the arms and legs. The weakness of the trunk favors progressive deformation of the spine such as scoliosis and kyphosis , which are also frequently described. The increased tension in the legs, which sometimes occurs and in people who are more neurologically affected, can contribute to problems with the hip ( hip dysplasia to hip subluxation).
Osteoporosis has been reported in some elderly people, but it is unclear whether this is a specific feature or a consequence of restricted mobility. An accumulation of bone fractures was not observed.
Dermatological features
Pathological sensitivity to light of the skin ( photosensitivity ) has long been considered the main characteristic of CS, mainly due to the underlying DNA repair defect and the resulting sensitivity of the cells to UV light. Clinically, however, only about a third to three quarters of those affected suffer from photosensitivity. They have a mild to severe sunburn after only a short exposure to the sun. The appearance of pigmented patches of skin on sun-exposed skin areas is described in some individuals . People with a CSA or CSB mutation are not at increased risk of developing skin cancer. Individuals with an additional mutation in an XP gene, who are also assigned to Cockayne syndrome, suffer from an extreme form of photosensitivity ( xeroderma pigmentosum ) and an increased risk of skin cancer .
In some cases, absent or reduced perspiration , dry, itchy skin, subtle dystrophy of the nails, atrophy of the skin, livedo reticularis and edema of the extremities have been reported.
Neurological features
There are a variety of neurological symptoms, which vary in severity in individual sufferers depending on the onset and severity of the disease. Hypertension of the limbs and spasms are seen in those with early onset of symptoms . As the neurological dysfunction increases, the speed of the tendon reflexes decreases. Symptoms that are triggered by changes in the cerebellum and occur in almost all those affected include disorders in movement coordination ( ataxia ) and muscle tremors ( tremor ). Ataxia and tremor can be the first signs of the disease if they initially develop normally. Seizures have been reported occasionally and are slightly more common than the general population. Once language has been acquired, language disorders can occur.
At the beginning of life, magnetic resonance imaging and computed tomography images of the brain are usually normal, even in those who are severely affected early on. Later, there may be calcification processes in various locations in the brain, white matter abnormalities (dysmyelination and hypomyelination), loss of white matter (demyelination), enlarged cerebral ventricles, and atrophy .
Developmental delay
The development of those affected is impaired to different degrees (see classification). The severity of the impairment is usually related to the overall severity of the disease. In the late stages of the disease, all those affected experience neurological degradation, which is associated with a reduction in cognitive abilities.
Liver, kidney and blood pressure
The liver enzymes of those affected are often slightly elevated ( transaminase increase ), but without any clinical significance. An enlarged liver has been reported occasionally.
Kidney problems have occasionally been reported but are not well documented. Occasionally, hypertension and proteinuria have been reported, which can lead to progressive kidney failure. Acute nephrotic syndrome and acute kidney failure have been reported sporadically in the late stages of the disease. It is often unclear whether it was caused by chronic kidney problems or by other pathological processes.
immune system
In general, those affected do not develop infections more frequently than the general population. However, in the advanced stages of the disease and symptoms such as high blood pressure or diabetes mellitus , the immune system can be impaired.
personality
As a rule, CS sufferers are described as very happy, open-minded and sociable.
More symptoms
Frequently persistent cold hands and feet regardless of the severity; Indigestion in the form of both constipation and thin stools; Sleep disorders (especially in those who are more neurologically affected); weak postpartum crying in individuals with early onset of symptoms; recurrent respiratory illnesses, often due to problems with swallowing and aspiration ; occasional changes in sugar metabolism and diabetes mellitus beginning in the second decade of life; occasionally atherosclerosis and circulatory disorders.
Progeria?
Some symptoms, such as loss of subcutaneous fat, progressive hearing loss, cognitive decline, kidney disease, atherosclerosis, chronic high blood pressure and diabetes are reminiscent of specific signs of normal aging and occur much earlier in people with CS than in the rest of the population. But all other neurological features, as well as degeneration of retinal pigmentation or specific anomalies of the teeth, do not belong to the usual clinical spectrum of aging. Whether these symptoms are the result of the same pathophysiological mechanisms in CS as in normal aging is not clear.
Classification
In the literature, a distinction is often made between three different types of Cockayne syndrome due to the variable onset and the different severity of the symptoms. However, it has proven to be difficult to clearly and unambiguously differentiate between the individual CS types, which is why CS can be described more as a continuous spectrum. For diagnosis, prognosis and for clinical studies, however, a classification seems to be useful in order to be able to divide those affected into largely homogeneous groups. But also within these groups different degrees of severity were described and individual individuals cannot always be clearly assigned to a certain type. It applies to all types that after an initial development and increase in skills and abilities, these level off at a certain level and then a loss of skills and abilities follows.
Type I or classic CS, also moderate / moderate CS:
After birth, weight, head circumference, and height are usually normal or less than normal. During the first or second year of life, the growth curve flattens out and leads to the small stature, microcephaly and low body weight typical of CS. People usually reach early developmental milestones such as spinning, sitting, and eating independently. However, motor and mental development is usually delayed. Individuals less severely affected learn to walk freely, while others cannot walk or can only walk a few steps. Progressive flexion contractures, scoliosis, kyphosis, ataxia, and tremors can hinder motor development and the ability to walk and stand is often lost as the disease progresses. Language development also usually begins later than usual. Those more severely affected often learn only a few words, while less severely affected individuals can form short sentences or communicate well with characters. Puberty usually begins at the usual time.
Type II or early-onset CS (early onset), also severe / severe CS:
Growth and weight gain are most limited when compared to the other types and plateau early. In some cases, too little growth ( SGA or IUGR ) and / or microcephaly is already observed during pregnancy. Special features include, among other things, early and increasing problems with food intake, difficulty swallowing and restricted development of motor and mental abilities. People who are severely affected cannot turn from back to stomach and neither learn to sit nor crawl. Less affected individuals reach these first milestones and usually lose the ability to sit freely again. Failure to learn or lose these basic motor skills is also promoted by weakness in the trunk, increased tension in the arms and especially the legs, progressive contractures of the joints, and kyphosis. Language development is usually very limited; some less severely affected learn individual words or characters.
Type III or mild / atypical CS:
The onset of the first symptoms, the course of the disease and life expectancy in this group vary the most between the individual affected individuals. The initial development is usually normal and the first symptoms only appear after the age of 3. Height and weight can be normal or even above average up to the first decade of life. The head circumference can be reduced a little, but still grow in the normal statistical range, but ultimately remains below the value of the average population. The head usually stops growing between the ages of 7 and 10. The fact that this type is considered rare or atypical could be due to the fact that those affected potentially get a late or even no CS diagnosis more often. With relatively normal motor development, those affected can run and, with a few exceptions, run and exercise. However, the development of fine motor skills is often limited by the early onset of tremor . Those affected usually learn to speak, read and write in full sentences. However, mental development is often restricted and cognitive deficits and decreased intelligence have been reported. Puberty occurs at the usual time and occasional successful pregnancies have been documented. After the initially appearing normal development, growth and weight gain begin to stagnate and there is often a loss of appetite and increasing difficulty swallowing. As the disease progresses, motor and mental abilities decline and other CS-typical symptoms such as partial or complete hearing loss and visual impairment appear. Early dementia, typically after the age of 30, has also been reported.
These classic subtypes have been expanded by two further types by various authors. The COFS syndrome can be viewed as a particularly severe variant of the CS syndrome, in which the symptoms manifest themselves before birth. In addition, there are individuals in whom the first symptoms do not appear until adulthood and cases have been described in which a CSB mutation was detected, but apart from an unusually strong sensitivity to UV light (UV-sensitive syndrome) no others for CSB Symptoms typical of the syndrome occur. These two variants can be viewed as the extreme ends of a continuous CS spectrum.
It has not been clarified whether there is a relationship between genotype and phenotype, i.e. whether a mutation in the CSA or CSB gene favors the development of a certain clinical picture or type. In the more severely and earlier affected, however, a CSB mutation appears to be somewhat more common and in the less affected, a CSA mutation. Other genetic and / or environmental factors could also play a role in the severity of the CS phenotype manifested in an individual.
It was noted that a classification does not necessarily make sense for the families of those affected, as the course can be very different for individual individuals (e.g. early onset, but rather slow progression of the symptoms) and an allocation of the sick child to one of the types described potentially creates uncertainty.
diagnosis
Photosensitivity of the skin has long been considered the most important diagnostic criterion. Before the discovery of the genes that are important for CS ( CSA and CSB ) in the mid-1990s, testing of the sensitivity of fibroblasts to UV-C radiation was exclusively used at the molecular level as evidence of CS disease. However, the role of this feature has been questioned and it has been noted that using this method alone as a diagnostic criterion has potentially overlooked some cases in the past. One study showed that only about a third of the affected individuals suffered from pronounced photosensitivity and about 1/5 showed no signs of photosensitivity. This agrees with reports by other authors who report an incidence of photosensitivity in people with CS of around two thirds to three quarters.
In general, it is often difficult to get an early diagnosis, since it is a disease that progresses and the onset and severity of the individual symptoms can vary greatly between affected individuals. Various authors have created lists of criteria that can be used for diagnosis.
| Diagnostic criteria | Nance and Berry, 1992 | Laugel, 2013 * | Wilson, 2016 ** |
|---|---|---|---|
| Main criteria
must be available |
- developmental delay
- stunted growth / short stature |
- developmental delay
- Progressive growth disorder - Progressive microcephaly |
- stunted growth
- microcephaly |
| Secondary criteria
(at least 3 of 5 / at least 3 of 5 / |
- Skin sensitivity to light
- Retinal degeneration ( retinitis pigmentosa ) and / or cataract - sensorineural hearing loss (sensorineural hearing) - Caries - Cachectic short stature |
- Skin sensitivity to light
- Retinal degeneration ( retinitis pigmentosa ) and / or cataract - sensorineural hearing loss (sensorineural hearing) - Sunken eyes ( enophthalmia ) |
- Persistently cold hands and feet
- Bilateral hearing loss - Skin sensitivity to light - (Intension) tremor - contractures of the joints - Progressive loss of subcutaneous adipose tissue - Cataract - typical facial features |
| Exclusion criteria /
CS diagnosis in question establishing criteria |
- Lack of microcephaly | - Malformation of the heart or kidneys |
* Laugel, 2013: In addition, the following imaging results can be helpful for the diagnosis: white matter hypomyelination and atrophy , cerebellar atrophy or hypoplasia and bilateral calcification of the putamen .
** Wilson, 2016: Developmental delay alone seems to be a rather weak criterion for diagnosis, especially for those with a late onset of symptoms. If the two main criteria and at least two of the secondary criteria are present, a DNA analysis should be carried out directly, if possible, since a skin biopsy must be performed to investigate the recovery of the RNA synthesis of the fibroblasts after UV radiation and the result is not always clear . Molecular genetic testing requires a less invasive procedure (blood or saliva sample) and provides a clear result.
treatment
At the current time (as of 2020) there is no causal treatment for the disease. Treatments aim to maintain the highest possible quality of life for those affected by CS and serve to avoid and alleviate painful symptoms.
The focus is usually on reduced weight gain, hearing and visual impairment, physiotherapeutic treatment and provision of aids.
Reduced weight gain
The growth and weight of children with CS often fall below the age-appropriate percentiles before a diagnosis is made. Once diagnosed, the decision on what action to take should be taken into account that slight weight gain and restricted growth are a fundamental, unchangeable part of the disease, and the decision to supplemental should be based on the clinical status of the individual.
Swallowing difficulties are common, especially in individuals more neurologically affected, and loss of appetite can occur in the advanced stages of the disease. In some cases, nutritional supplements such as normal or high-calorie drinking foods can be helpful. Young people affected may experience recurring vomiting, which requires repeated administration of small amounts. A nasogastric tube or a PEG tube can be helpful for those affected in the severely affected or in the advanced stages of the disease . This also facilitates the administration of medication. A breakdown of subcutaneous adipose tissue that has already started cannot be stopped by increasing the amount of food or calories. At this stage of the disease, the stomach appears to have difficulty adjusting to increased amounts of food, which in turn can lead to vomiting. An increase in the amount of food should therefore only take place moderately and carefully. In calorimetric measurements in a study that has not yet been published (as of 2020), it was observed that many sufferers have a lower calorie requirement (up to 1/3 less than usual for the corresponding weight or age group) and therefore the hypothesis that CS - Those affected generally have a reduced metabolism (hypometabolism).
digestion
CS sufferers often suffer from digestive disorders, which can manifest themselves in the form of constipation as well as thin stools. The former can be managed well with medication. In the case of recurring diarrhea, care must be taken to compensate for the increased loss of fluid and the risk of diaper rash .
Hearing and vision problems
It must be decided on an individual basis which treatment makes sense in the event of hearing and visual difficulties. People with CS are often given hearing aids, in some cases with cochlear implants . Cataracts can be surgically removed. Depending on the ophthalmological findings, glasses can be prescribed. It is generally recommended to protect your eyes by avoiding direct sun and wearing sunglasses outdoors.
Physiotherapeutic and orthopedic treatment
Due to the frequent problems of the musculoskeletal system, regular physiotherapeutic and orthopedic assessments are useful. Physiotherapy treatment should be tailored to the specific problems of the individual in order to counteract the deterioration in mobility, muscle breakdown and stiffening of the joints and muscles. The use of aids such as orthotics, therapy chairs, rehab buggies and standing stands to support the treatment and to make everyday life easier should be tailored to the needs of those affected.
Contractures of the legs can also lead to problems with the hips, which can make it difficult to care for affected children (e.g. swaddling by preventing the legs from splaying apart). Appropriate physiotherapeutic treatment and the use of aids can counteract this problem at an early stage. Sometimes the use of botox injections in the legs or surgery helped alleviate this problem. However, the expected advantages of invasive measures should be carefully considered and only carried out if absolutely necessary.
Skin sensitivity to light
There is also no causal treatment for any skin photosensitivity that may be present. In the case of particularly severely affected individuals, suitable preventive and therapeutic measures should be discussed with the treating physicians. For more easily affected individuals, simple measures such as suitable sun protection through appropriate clothing, wearing a sun hat and sun cream are usually sufficient.
tremor
The early occurrence of an intension tremor can impair fine motor development, activities such as playing or eating and thus the overall quality of life. Good reactions to levodopa / carbidopa have been reported. Whether drug treatment is an option has to be decided on an individual basis.
Dental problems
It has been observed that people with CS often suffer from tooth decay. This could possibly be the result of enamel hypolasia, decreased salivation, gastroesophageal reflux, increased vomiting or poor dental hygiene due to the neurological impairment. Regardless of the cause, it is important to know that if left untreated , tooth decay can lead to inflammation with severe pain and it makes sense to have the condition of the teeth checked regularly and, if necessary, to take preventive or treatment measures at an early stage.
Recommendations for medical care
In Germany, the ongoing care of affected children is optimally taken over by a local social pediatric center (SPZ) in addition to a resident pediatrician . It is recommended to carry out the following examinations at regular intervals in order to observe the progress and, if necessary, to take suitable measures for treatment in good time:
| system | examination | frequency |
|---|---|---|
| nutrition | Assessment of the nutritional status | Half-yearly |
| eyes | Cataracts and retinopathy | Every six months up to the age of 4, then annually |
| Hearing | Age-adjusted hearing tests | Yearly |
| Clinical examinations of the cardiovascular system, liver, kidneys, metabolism | - Blood pressure, further examinations if symptomatic
- liver enzymes - kidney function; Uric acid & proteinuria blood sugar levels (from the age of 10) |
Yearly |
| Nervous system | Neurological / neuropediatric assessment | Half-yearly |
| Musculoskeletal system | Orthopedic and physiotherapeutic assessment | Semi-annually / annually |
Medication and inpatient treatment
It has been observed that the administration of metronidazole causes acute liver failure, which can be fatal. Related antibiotics should also be avoided as a precaution.
In individual cases, pronounced reactions to sedatives and opioids have been reported - this ranges from respiratory depression to emotional numbness for several days after the administration of codeine. For opioids, it is recommended to start with a third of the regular dose if necessary.
Other drugs should always be dosed according to weight and not age.
Most people with CS have poor peripheral blood flow and venous access can be difficult; therefore it is recommended that this is only carried out by particularly experienced persons. Problems with intubation have also been reported. Whenever possible, adults with CS should be treated in a pediatric department due to their small stature and low weight (often only 10–17 kg).
Excessive intravenous or rapidly increasing fluids can lead to overhydration with serious health consequences. It is recommended that intravenous fluid administration not be based on age or weight tables, but rather on the previous fluid intake and only increase the fluid intake very slowly and with good monitoring.
forecast
Due to the progressive course of the disease and the lack of treatment options for the cause of CS, the life expectancy of affected individuals is limited to different degrees. Due to the high variability, it is difficult to give a general prognosis for individual individuals.
In a systematic review from 1992, the average age of 37 subjects at death was 12 years; Of these, three were less than 2 years old and two were older than 30 years; In a 2016 survey, the average age of 28 people at death was 8.4 years with a range from 17 months to 30 years. In both studies, the occurrence of cataracts before the age of three was shown to be the most important prognostic factor for a low life expectancy. In another study with 45 individuals, however, no such connection was found; although cataracts were more common in severely affected individuals, two of the oldest study participants (42 and 44 years old) had cataracts at birth.
Broken down according to the three different degrees of severity of CS, the average life expectancy for people with type I was 16.1 years (range 11–22 years), type II with 5 years (range 0.6–11 years) and type III with 30.3 years (range 22–42 years). However, there are also reports in the literature of slightly affected individuals who lived to be 50 years or older.
The multiple causes of death include pneumonia / respiratory disease, respiratory failure, kidney or liver failure, heart failure, complications from seizures, stroke and other complications of the various symptoms of the disease.
frequency
The incidence ( incidence ) of CS in Western Europe is estimated at 2.7 per million births, but it could be higher. One reason for a possible underestimation of the frequency of occurrence in the past could be in general to the advancement of diagnostic procedures and the fact that the photosensitivity of the skin was long considered the most important diagnostic characteristic, whereby some cases without this characteristic were possibly overlooked. Even in cases with a late onset of symptoms, it can happen that CS remains undetected as the cause of the symptoms for a long time.
In a study in Japan, the prevalence was estimated at 1 in 2.5 million. Female and male individuals are affected equally often and CS is widespread worldwide, with regional clusters being observed in some cases.
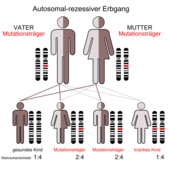
Risk to family members
The parents of affected children are heterozygous carriers of a pathogenic variant of the CSA / ERCC8 or CSB / ERCC6 gene. You are asymptomatic and have no risk of developing the disease yourself. The siblings of those affected and the siblings of the parents have a 50% risk of being asymptomatic carriers themselves. 25% of the siblings of those affected are not affected themselves and also no carriers. It is not known that people with type I or II CS have had children themselves. The children of type III patients with CS are 100% heterozygous carriers of a pathogenic variant of the CSA / ERCC8 or CSB / ERCC6 gene.
research
The disease and its causes have gained attention in specialist circles over the past few decades. In Germany, research is currently being carried out into whether the administration of artificially produced chaperones can positively influence the course of the disease. So far, however, this has only been tested on mice and there are no clinical studies on humans (as of 2020).
At the University of Cologne, the effects of the genetic defect responsible for CS were simulated on the model of roundworms in order to better understand the underlying mechanisms and to investigate possible pharmacological treatments.
Further information
- Amy and friends is a UK network for those affected, their families and loved ones from Cockayne Syndrome and Trichothiodystrophy.
- Share and Care Cockayne Syndrome Network is an American network for those affected, their families and relatives.
Both networks regularly organize conferences for families, their relatives and treating professionals with international guests.
Differential diagnosis
Among other things, the Flynn-Aird syndrome must be distinguished .
literature
- G. Hirschfeld, M. Berneburg, C. Arnim et al: Progeroid Syndrome: Clinical and molecular biology of premature aging. In: Deutsches Ärzteblatt. Volume 104, Number 6, 2007, pp. A-346 / B-305 / C-292
- X. Yuan, W. Feng et al: Activation of RNA polymerase I transcription by cockayne syndrome group B protein and histone methyltransferase G9a. In: Molecular cell. Volume 27, Number 4, August 2007, pp. 585-595, ISSN 1097-2765 . doi: 10.1016 / j.molcel.2007.06.021 . PMID 17707230 .
- C. Ovaert, A. Cano, B. Chabrol: Aortic dilatation in Cockayne syndrome. In: American journal of medical genetics. Part A. Volume 143A, Number 21, November 2007, pp. 2604-2606, ISSN 1552-4825 . doi: 10.1002 / ajmg.a.31986 . PMID 17935247 .
Web links
- Cockayne Syndrome. In: Orphanet (Rare Disease Database).
- Cockayne syndrome type A. In: Online Mendelian Inheritance in Man . (English)
- Cockayne syndrome type B. In: Online Mendelian Inheritance in Man . (English)
- Cockayne syndrome type C. In: Online Mendelian Inheritance in Man . (English)
- cockaynesyndrome.net
- Genes and Disease: Article Collection
Individual evidence
- ↑ a b c d e f Martha A. Nance, Susan A. Berry: Cockayne syndrome: Review of 140 cases . In: American Journal of Medical Genetics . tape 42 , no. 1 , 1992, ISSN 1096-8628 , pp. 68-84 , doi : 10.1002 / ajmg.1320420115 .
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae Brian T. Wilson, Zornitza Stark, Ruth E. Sutton, Sumita Danda, Alka V Ekbote: The Cockayne Syndrome Natural History (CoSyNH) study: clinical findings in 102 individuals and recommendations for care . In: Genetics in Medicine . tape 18 , no. 5 , May 2016, ISSN 1530-0366 , p. 483-493 , doi : 10.1038 / gim.2015.110 , PMID 26204423 .
- ↑ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an Vincent Laugel: Cockayne syndrome: The expanding clinical and mutational spectrum . In: Mechanisms of Aging and Development (= Special Issue on the segmental progeria Cockayne syndrome ). tape 134 , no. 5 , May 1, 2013, ISSN 0047-6374 , p. 161–170 , doi : 10.1016 / j.mad.2013.02.006 .
- ↑ L. Pasquier, V. Laugel, L. Lazaro, H. Dollfus, H. Journel: Wide clinical variability among 13 new Cockayne syndrome cases confirmed by biochemical assays . In: Archives of Disease in Childhood . tape 91 , no. 2 , February 1, 2006, ISSN 0003-9888 , p. 178-182 , doi : 10.1136 / adc.2005.080473 , PMID 16428367 .
- ↑ a b c d e f g h i j k l m n o p q r Valerie Natale: A comprehensive description of the severity groups in Cockayne syndrome . In: American Journal of Medical Genetics Part A . tape 155 , no. 5 , 2011, ISSN 1552-4833 , p. 1081-1095 , doi : 10.1002 / ajmg.a.33933 .
- ↑ EA Cockayne: Dwarfism with retinal atrophy and deafness . In: Archives of Disease in Childhood . tape 11 , no. 61 , February 1, 1936, ISSN 0003-9888 , p. 1-8 , doi : 10.1136 / adc.11.61.1 , PMID 21032019 .
- ↑ EA Cockayne: Dwarfism with Retinal Atrophy and Deafness . In: Archives of Disease in Childhood . tape 21 , no. 105 , March 1946, ISSN 0003-9888 , p. 52-54 , PMC 1987981 (free full text).
- ^ Catherine A. Neill, Mary M. Dingwall: A Syndrome Resembling Progeria: A Review of Two Cases . In: Archives of Disease in Childhood . tape 25 , no. 123 , September 1, 1950, ISSN 0003-9888 , p. 213-223 , doi : 10.1136 / adc.25.123.213 , PMID 14783428 .
- ↑ Debora Ferri, Donata Orioli, Elena Botta: Heterogeneity and overlaps in nucleotide excision repair disorders . In: Clinical Genetics . tape 97 , no. 1 , 2020, ISSN 1399-0004 , p. 12-24 , doi : 10.1111 / cge.13545 .
- ↑ Alain J. van Gool, Gijsbertus TJ van der Horst, Elisabetta Citterio, Jan HJ Hoeijmakers: Cockayne syndrome: defective repair of transcription? In: The EMBO Journal . tape 16 , no. 14 , July 15, 1997, ISSN 0261-4189 , p. 4155-4162 , doi : 10.1093 / emboj / 16.14.4155 , PMID 9250659 .
- ↑ Ajoy C. Karikkineth, Morten Scheibye-Knudsen, Elayne Fivenson, Deborah L. Croteau, Vilhelm A. Bohr: Cockayne syndrome: Clinical features, model systems and pathways . In: Aging Research Reviews (= Monogenic Accelerated Aging Disorders with Perturbations to Normal DNA and Chromosome Function ). tape 33 , January 1, 2017, ISSN 1568-1637 , p. 3–17 , doi : 10.1016 / j.arr.2016.08.002 , PMID 27507608 .
- ↑ Morten Scheibye-Knudsen, Deborah L. Croteau, Vilhelm A. Bohr: Mitochondrial deficiency in Cockayne syndrome . In: Mechanisms of Aging and Development (= Special Issue on the segmental progeria Cockayne syndrome ). tape 134 , no. 5 , May 1, 2013, ISSN 0047-6374 , p. 275–283 , doi : 10.1016 / j.mad.2013.02.007 , PMID 23435289 .
- Jump up ↑ Agnès Bloch-Zupan, Morgan Rousseaux, Virginie Laugel, Matthieu Schmittbuhl, Rémy Mathis: A possible cranio-oro-facial phenotype in Cockayne syndrome . In: Orphanet Journal of Rare Diseases . tape 8 , no. 1 , January 14, 2013, ISSN 1750-1172 , p. 9 , doi : 10.1186 / 1750-1172-8-9 , PMID 23311583 .
- ^ V. Laugel, C. Dalloz, M. Durand, F. Sauvanaud, U. Kristensen: Mutation update for the CSB / ERCC6 and CSA / ERCC8 genes involved in Cockayne syndrome . In: Human Mutation . tape 31 , no. 2 , 2010, ISSN 1098-1004 , p. 113-126 , doi : 10.1002 / humu.21154 .
- ↑ Metabolic Study of Cockayne Syndrome (METABO-CS). In: US National Library of Medicine. February 6, 2017, accessed May 31, 2020 .
- ^ A b Vincent Laugel: Cockayne Syndrome . In: GeneReviews® . University of Washington, Seattle, Seattle (WA) 1993, PMID 20301516 ( nih.gov [accessed May 31, 2020]).
- ^ Brian T. Wilson, Andrew Strong, Sean O'Kelly, Jennifer Munkley, Zornitza Stark: Metronidazole Toxicity in Cockayne Syndrome: A Case Series . In: Pediatrics . tape 136 , no. 3 , September 1, 2015, ISSN 0031-4005 , p. e706 – e708 , doi : 10.1542 / peds.2015-0531 , PMID 26304821 .
- ^ A b Isabelle Rapin, Karen Weidenheim, Yelena Lindenbaum, Pearl Rosenbaum, Saumil N. Merchant: Cockayne Syndrome in Adults: Review With Clinical and Pathologic Study of a New Case . In: Journal of Child Neurology . tape 21 , no. November 11 , 2006, ISSN 0883-0738 , p. 991-1006 , doi : 10.1177 / 08830738060210110101 .
- ↑ Fluid overload | Share and Care Network. Retrieved May 31, 2020 (American English).
- ↑ a b Masaya Kubota, Sayaka Ohta, Aki Ando, Akiko Koyama, Hiroshi Terashima: Nationwide survey of Cockayne syndrome in Japan: Incidence, clinical course and prognosis . In: Pediatrics International . tape 57 , no. 3 , 2015, ISSN 1442-200X , p. 339-347 , doi : 10.1111 / ped.12635 .
- ↑ a b Wim J. Kleijer, Vincent Laugel, Mark Berneburg, Tiziana Nardo, Heather Fawcett: Incidence of DNA repair deficiency disorders in western Europe: xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy . In: DNA Repair . tape 7 , no. 5 , May 3, 2008, ISSN 1568-7864 , p. 744-750 , doi : 10.1016 / j.dnarep.2008.01.014 .
- ↑ Marius Costel Alupei, Pallab Maity, Philipp Ralf Esser, Ioanna Krikki, Francesca Tuorto: Loss of Proteostasis Is a Pathomechanism in Cockayne Syndrome . In: Cell Reports . tape 23 , no. 6 , May 8, 2018, ISSN 2211-1247 , p. 1612–1619 , doi : 10.1016 / j.celrep.2018.04.041 , PMID 29742419 .
- ↑ Annika Bingmann: Cockayne Syndrome: Aging like in time lapse New therapeutic approach for "old children". May 8, 2018, accessed May 31, 2020 .
- ↑ Björn Schumacher receives Eva Luise Köhler Research Award 2019: Rare diseases provide insight into the secret of aging. February 18, 2019, accessed May 31, 2020 .
- ↑ What happens in Cockayne Syndrome. February 15, 2019, accessed May 31, 2020 .
- ↑ https://www.amyandfriends.org/
- ↑ http://cockaynesyndrome.org/