Deltamethrin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
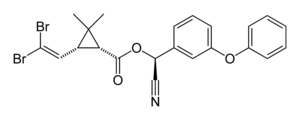
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Deltamethrin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 22 H 19 Br 2 NO 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action |
Opening of the Na + channels |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 505.21 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.5 g cm −3 |
|||||||||||||||
| Melting point |
98-101 ° C |
|||||||||||||||
| boiling point |
300 ° C |
|||||||||||||||
| solubility |
almost insoluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Deltamethrin is an insecticide from the group of pyrethroids and is used in veterinary medicine against various ectoparasites . It is also used as a plant protection product and for impregnating mosquito nets.
It was developed by the group of Michael Elliott ( Rothamsted Research ) and is a Type II pyrethroid .
Mode of action
Insects ingest deltamethrin through the surface of the body, whereupon it is distributed throughout the insect's body. It is a neurotoxin and causes the Na + channels of the nerve cells to no longer close. Na + ions flow unhindered into the inside of the cell and uncontrollable nerve impulses occur. This initially leads to states of excitement with cramps, then to coordination disorders and finally to paralysis. The insect is unable to move within a few minutes, which is known as a " knock-down " effect. Death only occurs after a while.
If the dose is insufficient, many of the insects affected can break down deltamethrin enzymatically (detoxification esterases and mixed function oxidase ). By adding synergists such as piperonyl butoxide , enzymatic degradation can be prevented.
application areas
In dogs, it is primarily used against fleas , ticks and the sand flies that transmit leishmania . It is applied locally to the skin using prepared flea collars or shampoos and spreads over the entire animal with the film of fat. There is no absorption through the skin.
According to Regulation (EEC) No. 2377/90 on maximum levels for veterinary drug residues in food , the active ingredient is also approved for use in sheep and cattle. Here, Deltamethrin is applied to the back as a solution (so-called pour-on method) and works against hair lice , lice and stinging and non-stinging willow flies. Because of the broad spectrum of activity of the pyrethroids, it is also assumed that they have an effect on midges , the carriers of bluetongue disease .
A preparation containing deltamethrin is approved as a pesticide in Germany and Austria for the cultivation of grain, rape, beet and potatoes as well as in meadows against various "biting" insects. Several preparations are approved in Switzerland, the range of applications is somewhat broader and includes, among other things, the use against bark beetles in felled wood.
In accordance with European legislation (Directive 98/8 / EC on the placing of biocidal products on the market) and with the resolution of September 20, 2011, a decision has been made to add the active ingredient deltamethrin to the corresponding list from October 1, 2013 (Annex I of Directive 98 / 8 / EG) for product type 18 (insecticides). The dispensing of biocides with the active ingredient deltamethrin is therefore initially still permitted in the EU until September 30, 2023. Switzerland has adopted this provision.
Deltamethrin can also be used to impregnate mosquito nets .
Residues in food
In Switzerland there is a relatively high maximum residue level of 2 milligrams of deltamethrin per kilogram for grain .
Trade names
- Butox, Decis, Deltamax, Deltatic, K-Obiol, K-Othrine, Latroxin Delta, Prevendog, Scalibor, Scatto
Individual evidence
- ↑ a b c d e f g Entry on Deltamethrin in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ entry to deltamethrin in Vetpharm, accessed on 21 November 2011th
- ↑ Entry on α-cyano-3-phenoxybenzyl [1R- [1α (S *), 3α]] - 3- (2,2-dibromovinyl) -2,2-dimethylcyclopropanecarboxylate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA) , accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ FLI: Bluetongue Disease - Recommendations for the Protection of Ruminants from Infestation with Midges. Sept. 2007 pdf
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Deltamethrin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 13, 2016.
- ↑ EU: Directive 98/8 / EC of February 16, 1998 on the placing of biocidal products on the market (PDF) Official Journal of the European Communities L 123/1 of April 24, 1998.
- ↑ EU: Directive 2011/81 / EC of September 20, 2011 amending Directive 98/8 / EC to include deltamethrin in Appendix I (PDF) Official Journal of the European Communities L 243/16 of September 21, 2011.
- ↑ Thomas Morwinsky: Tips on mosquito and tick protection , General Practitioner online, May 25, 2013, accessed on December 16, 2017.
- ↑ Ordinance of the EDI on the maximum levels for pesticide residues in or on products of plant and animal origin. In: admin.ch . Retrieved February 6, 2020 .

