Diisopropanolamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
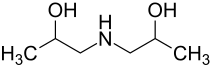
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Diisopropanolamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 15 NO 2 | ||||||||||||||||||
| Brief description |
colorless solid with an amine- like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 133.19 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.99 g cm −3 |
||||||||||||||||||
| Melting point |
42 ° C |
||||||||||||||||||
| boiling point |
249 ° C |
||||||||||||||||||
| Vapor pressure |
0.02 hPa (20 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Diisopropanolamine ( 1,1'-iminodipropan-2-ol ) is a chemical compound from the group of amino compounds and alcohols , more precisely the alkanolamines .
Extraction and presentation
Diisopropanolamine can be obtained by reacting isopropanolamine or ammonia with propylene oxide .
properties
Diisopropanolamine is a flammable, hygroscopic, colorless solid with an amine-like odor, which is easily soluble in water. When exposed to light and air, the compound turns yellowish. The flash point is 135 ° C, the ignition temperature is 290 ° C and the explosion limits are between 1.6% by volume (lower explosion limit) and 8.0% by volume (upper explosion limit).
use
Diisopropanolamine is used in the manufacture of emulsifiers , absorbents, cosmetic preparations and foam stabilizers . Isopropanolamines, including diisopropanolamine, neutralize acidic components in cosmetic products and thus allow a balanced pH value in hairsprays, creams, skin lotions and moisturizers. In the metal processing industry it is used as a corrosion protection agent and in the cement industry as a setting accelerator. It is also used in the chemical separation of carbon dioxide and hydrogen sulfide from natural gas in the sulfinol process.
Risk assessment
Diisopropanolamine was included in the EU's ongoing action plan ( CoRAP ) in 2013 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. The reasons for the intake of diisopropanolamine were concerns about consumer use , exposure of workers , high risk characterization ratio (RCR) and widespread use, as well as the dangers arising from a possible assignment to the group of CMR substances and the possible danger from sensitizing properties . The re-evaluation took place from 2013 and was carried out by the Czech Republic . A final report was then published.
Individual evidence
- ↑ a b c d e f g h i j k Entry on 1,1′-iminodipropan-2-ol in the GESTIS substance database of the IFA , accessed on December 22, 2019(JavaScript required) .
- ↑ Entry on Diisopropanolamine (mixture of isomers) at TCI Europe, accessed on June 27, 2011.
- ↑ Entry on 1,1′-iminodipropan-2-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ a b Canadian Soil and Water Quality Guidelines for Diisopropanolamine (Canadian Council of Ministers of the Environment 2006) (PDF; 703 kB)
- ↑ Ken Arnold and Maurice Stewart; Surface Production Operations: Design of gas-handling systems and facilities; ISBN 978-0-88415-822-6 .
- ^ European Chemicals Agency (ECHA): Substance Evaluation Report and Conclusion Document .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): 1,1'-iminodipropan-2-ol , accessed on March 26, 2019.


