Enterobacteriophage MS2
| Enterobacteriophage MS2 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
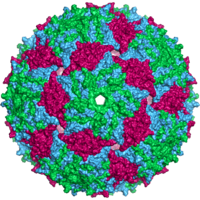
Bacteriophage MS2 capsid |
||||||||||||||||||
| Systematics | ||||||||||||||||||
|
||||||||||||||||||
| Taxonomic characteristics | ||||||||||||||||||
|
||||||||||||||||||
| Scientific name | ||||||||||||||||||
| Escherichia virus MS2 | ||||||||||||||||||
| Short name | ||||||||||||||||||
| MS2 | ||||||||||||||||||
| Left | ||||||||||||||||||
|
The Enterobakteriophage MS2 , officially Escherichia virus MS2 , outdated Enterobacteria phage MS2 , German and bacteriophage MS2 or coliphage MS2 , named MS2, is an icosahedral single-stranded RNA virus with positive polarity , of the bacterium Escherichia coli and other members of the Enterobacteriaceae infected.
Phage MS2 belongs to a family of closely related bacterial viruses that also includes Enterobacteriophage Qβ (aka Escherichia virus Qbeta ) and Enterobacteriophage BZ13 (aka Enterobacteriophage BZ13 ).
Research history
In 1961, MS2 was isolated by Alvin John Clark and recognized as phage containing RNA. In 1976 the MS2 genome was the first virus genome ever to be completely sequenced. This was achieved by Walter Fiers and his team, who had already completely sequenced the first gene in 1972 with the gene for the MS2 envelope protein. The first complete determination of a DNA genome took place later, in 1977 with that of the enterobacteriophage ΦX174 .
The first attempt at statistical analysis of the MS2 genome was to look for patterns in the nucleotide sequence . Several non-coding sequences (gene segments that are not translated into proteins) were identified, although at the time of this investigation (1979) the function of the non-coding patterns was still unknown.
Genome
The MS2 genome is one of the smallest known genomes, consisting of 3569 nucleotides of single-stranded RNA.
It encodes only four proteins: the maturation protein (A protein), the lysis protein, the coat protein and the replicase protein . The gene lys , which codes for the lysis protein, overlaps both the 3 'end of the upstream gene cp and the 5' end of the downstream gene rep and was one of the first known examples of overlapping genes.
The positive-stranded RNA genome serves as messenger RNA and is translated after the virus has been stripped of its envelope in the host cell.
Although the four proteins (i. E. Viral RNA) by the same messenger RNA encoded, they are not all at the same levels expressed (for details see. U.).
The expression of these proteins is regulated by a complex interplay between translation and RNA secondary structure .
Capsid structure
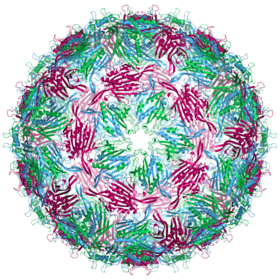
The MS2 virion (virus particle) is approximately 27 nm in diameter as determined by electron microscopy.
It consists of one copy of the maturation protein and 180 copies of the envelope protein (organized in 90 dimers ) arranged in an icosahedral envelope with the triangulation number T = 3 to protect the RNA genome inside.
The structure of the coat protein is a five-stranded β-sheet with two α-helices and a hairpin . After the capsid has been assembled, the helices and the hairpin face the outside of the virus particle, while the β-sheet faces the inside.
Mode of action
MS2 infected enterobacteria that the fertility factor F comprise. This is a plasmid with which the bacterial cells can serve as DNA donors during bacterial conjugation . Genes on the F plasmid lead to the production of an F pilus (also called sex pilus ), which serves as a viral receptor (DNA receiver). MS2 binds to the side of the pilus through its only maturation protein. The exact mechanism by which phage RNA enters the bacterium is still unknown.
Once the viral RNA has entered the cell, it begins to act as messenger RNA for the production of phage proteins. The gene cp for the most common protein, the envelope protein, can be translated immediately. The translation start ( start codon ) of the replica gene rep , on the other hand, is normally hidden in the RNA secondary structure, but can be temporarily opened when ribosomes run through the gene of the coat protein. Replicase translation is also terminated as soon as large amounts of coat protein have been produced. Dimers of the coat protein bind and stabilize the RNA operator hairpin, whereby the start of replicase is blocked. The start of the maturation protein gene mat is accessible in the RNA, which is replicated but is hidden in the RNA secondary structure of the finished MS2 RNA. This ensures the translation of only a very few copies of the maturation protein per RNA. Finally, the translation of the lysis protein gene can only be started by ribosomes that have completed the translation of the coat protein gene .
This ensures that the individual sub-steps of virus replication (RNA replication, formation of the coat protein and finally lysis (dissolution) of the host cell) take place in the correct order.
The replication of the plus strand of the MS2 genome requires the synthesis of the complementary minus strand RNA, which can then be used as a template for the synthesis of a new plus strand RNA. Because MS2 replicase is difficult to isolate, MS2 replication has been studied much less well than replication of the closely related bacteriophage Qβ , but appears to be very similar.
It is believed that the formation of the virion is triggered by the binding of maturation protein to the MS2 RNA. The complex of ripening protein and RNA has already been proven to be infectious. The icosahedral shell (the capsid) can also be built up from coat proteins in the absence of RNA; the capsid is arranged by attaching the coat protein dimer to the RNA operator hairpin, so that the overall structure is nucleated (with the virus RNA) becomes. If MS2-RNA is present, assembly takes place even at much lower concentrations of coat protein.
Bacterial lysis and the release of newly formed virions happens when enough lysis protein has accumulated. The lysis protein forms pores in the cytoplasmic membrane (bacterial envelope), which leads to the loss of the membrane potential and the breakdown of the cell wall .
Applications
The MS2 operator hairpin and envelope protein have been used for the detection of RNA in living cells since 1998 (MS2 tagging).
MS2 was used as a replacement for norovirus in disease transmission studies because of its structural similarities to noroviruses , its similarly good conditions for proliferation, and its non- pathogenicity in humans.
See also
literature
- J. Glasgow, D. Tullman-Ercek: Production and applications of engineered viral capsids . In: Appl. Microbiol. Biotechnol. , 98, 2014, pp. 5847-5858, doi: 10.1007 / s00253-014-5787-3
Web links
- Complete genome (also isolates R17 , DL16 , and J20 )
Individual evidence
- ↑ ICTV Master Species List 2018b v1 MSL # 34, Feb. 2019
- ↑ a b c d ICTV: ICTV Master Species List 2019.v1 , New MSL including all taxa updates since the 2018b release, March 2020 (MSL # 35)
- ↑ a b c d e f g J. van Duin, N. Tsareva: Single-stranded RNA phages. Chapter 15 . In: RL Calendar (Ed.): The Bacteriophages , Second. Edition, Oxford University Press, 2006, ISBN 0-19-514850-9 , pp. 175-196.
- ↑ CZ Ni et al .: Crystal structure of the coat protein from the GA bacteriophage: model of the unassembled dimer . In: Protein Sci. , 5, pp. 2485–2493, 1996, PMC 2143325 (free full text)
- ↑ JE Davis, JH Strauss, RL Sinsheimer: Bacteriophage MS2: another RNA phage . In: Science . 134, No. 3488, 1961. doi : 10.1126 / science.134.3488.1425 . P. 1427
- ↑ a b W. Fiers, R. Contreras, F Duerinck, G. Haegeman, D. Iserentant, J. Merregaert, W. Min Jou, F. Molemans, A. Raeymaekers, A. Van den Berghe, G. Volckaert, M Ysebaert: Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene . In: Nature . 260, No. 5551, 1976, pp. 500-507. doi : 10.1038 / 260500a0 . PMID 1264203 .
- ^ W Min Jou, G. Haegeman, M. Ysebaert, W. Fiers: Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein . In: Nature . 237, No. 5350, 1972, pp. 82-88. doi : 10.1038 / 237082a0 . PMID 4555447 .
- ↑ FF Sanger et al : Nucleotide sequence of bacteriophage φX174 DNA . In: Nature . 265, No. 5596, 1977, pp. 687-695. doi : 10.1038 / 265687a0 . PMID 870828 .
- ↑ A search for patterns in the nucleotide sequence of the MS2 genome, Erickson, JW and Altman, GG; J. Mathematical Biology, April 1979, Volume 7, pp219-230
- ^ JH Strauss, RL Sinsheimer: Purification and properties of bacteriophage MS2 and of its ribonucleic acid . In: J Mol Biol . 7, No. 1, 1963, pp. 43-54. doi : 10.1016 / S0022-2836 (63) 80017-0 .
- ↑ K. Valegård, L. Lilias, K. Fridborg, T. Unge: The three-dimensional structure of the bacterial virus MS2 . In: Nature . 345, No. 6270, 1990, pp. 36-41. doi : 10.1038 / 345036a0 .
- ↑ R. Golmohammadi, K. Valegård, K. Fridborg, L. Liljas: resolution The refined structure of bacteriophage MS2 at 2 · 8 Å . In: J Mol Biol . 234, No. 3, 1993, pp. 620-639. doi : 10.1006 / jmbi.1993.1616 .
- ↑ E. Bertrand, P. Chartrand, M. Schaefer, SM Shenoy, RH Singer, RM Long: Localization of ASH1 mRNA particles in living yeast . In: Mol. Cell , 2, 1998, pp. 437-445.
- ↑ Maggie Fox: Viruses spread 'like crazy' in an office, study finds .
