Forchlorfenuron
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
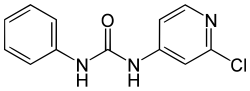
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Forchlorfenuron | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 10 ClN 3 O | ||||||||||||||||||
| Brief description |
white odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 247.68 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.44 g cm −3 |
||||||||||||||||||
| Melting point |
165 ° C |
||||||||||||||||||
| Vapor pressure |
4.6 hPa (26 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Forchlorfenuron is a chemical compound from the group of urea derivatives , nitrogen heterocycles , substituted phenols and organic chlorine compounds that is used as an active ingredient in pesticides . Today it is made by AlzChem .
properties
Forchlorfenuron is a flammable white odorless solid that is insoluble in water.
use
Forchlorfenuron acts as a growth regulator in plants . The active ingredient belongs to the cytokinins and thus to the phytohormones .
Forchlorfenuron increases the fruit size in kiwis, table grapes and peaches, increases the fruit set in melons and cucumbers and increases the yield of tomatoes, rice and cereals.
The active ingredient became known in 2011 through a report on Chinese television about exploding watermelons caused by improper use of forchlorfenuron.
Admission
In some EU countries, plant protection products with this active ingredient are approved, but not in Germany, Austria or Switzerland.
Web links
Individual evidence
- ↑ a b c d e f g h Entry on forchlorfenuron in the GESTIS substance database of the IFA , accessed on February 5, 2017(JavaScript required) .
- ↑ a b Data sheet forchlorfenuron from Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ Forchlorfenuron data sheet (PDF file; 58 kB) from Bio Basic, accessed January 3, 2013.
- ↑ Entry on 1- (2-chloro-4-pyridyl) -3-phenylurea in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ AlzChem AG
- ↑ Entry on forchlorfenuron. In: Römpp Online . Georg Thieme Verlag, accessed on June 17, 2014.
- ↑ China: Exploding melons horrify farmers. In: Spiegel Online . May 17, 2011, accessed May 18, 2011 .
- ^ Directorate-General for Health and Food Safety of the European Commission: Entry on forchlorfenuron in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 25, 2016.

