Hypertrophic cardiomyopathy
The hypertrophic cardiomyopathy (also: hypertrophic cardiomyopathy; HCM, formerly idiopathic hypertrophic subaortale stenosis, IHSS) is a monogenic hereditary disease and belongs to the large group of cardiomyopathies ( Greek καρδία cardia , German , heart ' , gr μυς. Mys , muscular' , gr. πάθος páthos 'suffering'; disease of the heart muscles). It is characterized by a mostly asymmetrical thickening ( hypertrophy ) of the muscles of the left ventricle over 15 mm without adequate pressure load and by a disordered arrangement of the myocytes (disarray). In some cases, the left-sided outflow tract becomes narrower ( obstruction ) under stress ( hypertrophic obstructive cardiomyopathy , HOCM) and, in the course, the heart muscle stiffens (with reduced elasticity) ( compliance disorder ). The main complaints are shortness of breath under exertion and sometimes dangerous cardiac arrhythmias . The disease is treated with drugs that lower the heart's strength and, in the case of an obstruction of the outflow tract, with cardiac catheter-guided or surgical muscle removal.
| Classification according to ICD-10 | |
|---|---|
| I42.1 | Hypertrophic obstructive cardiomyopathy |
| I42.2 | Hypertrophic non-obstructive cardiomyopathy |
| ICD-10 online (WHO version 2019) | |
Cause - etiology
The disease is congenital and occurs in families. It is inherited as an autosomal dominant trait. Women and men are equally affected. The prevalence is at least 1: 500. This makes HCM the most common heart disease caused by a genetic defect.
genotype
Over 1,500 gene defects (mutations) in over 27 gene loci , which predominantly encode proteins of the cardiac sarcomere , are known. The main defects (over 50%) are in the structure of the beta-myosin heavy chain (a myosin filament , the first identified gene defect) in myosin binding protein C and Troponin-T . Sedaghat-Hamedani et al. have shown in a large meta-analysis that patients with an MYH7 mutation have a worse prognosis than HCM patients without such mutations.
Phenotype
The phenotypic expression is usually not exclusively dependent on a single mutation, but is also determined by “modifier genes” and environmental factors. The clinical picture ( left ventricular hypertrophy in echocardiography , EKG changes) may be completely absent, despite a proven genetic defect. Children under the age of 13 are usually the only "silent carriers".
Fine tissue changes - pathology
The tissue picture is not specific. There is a branching disorder of the hypertrophied heart muscle cells with a lack of the normal parallel arrangement due to increased lateral branches that have been replaced by side-to-side connections (myocytic disarray). There is also a connective tissue ( fibrotic ) remodeling of the interstitium . The microscopic changes are not limited to the macroscopically hypertrophied areas. A similar picture, but not nearly as pronounced, can also be seen in other diseases associated with cardiac muscle hypertrophy.
Disease origination - pathogenesis
In about two thirds of affected patients, the myocardial thickening is located in the outflow tract of the left ventricle, which, depending on the degree of severity, leads to a narrowing (obstruction) of the outflow tract under stress (physical or medicinal) or at rest. This creates a functional aortic stenosis with increased pressure on the left ventricle. HCM is accordingly divided into an obstructive (HOCM) and a non-obstructive form (HNCM) . In the case of obstruction that can only be detected under stress, it is also referred to as HCM with dynamic obstruction . About 12% of patients with HOCM suffer from an atypical form in which the obstruction is not subaortic but mid-ventricular.
In addition to the narrowing of the outflow tract in HOCM, muscle thickening leads to muscle stiffening in both forms of HCM. As a result, the heart chamber can only fill up to a limited extent in its relaxation phase ( diastole ), as a result of which the blood backs up in the pulmonary veins with subsequent shortness of breath. One speaks of a diastolic heart failure (diastolic compliance disorder ). In HOCM, muscle stiffening increases due to the increased work (pumping power against the narrowness of the outflow tract).
Furthermore, with HOCM, due to the flow acceleration occurring in the area of the narrowed outflow tract, a suction effect on the mitral valve ( Venturi effect ), which can usually only leak moderately ( mitral insufficiency ).
The main problem in addition to the restriction of exertion is the tendency to serious arrhythmias , especially those occurring under exertion . These can be accompanied by syncope (brief loss of consciousness) and sudden cardiac death . For example, sudden deaths from exercise in people under the age of 35 are often caused by HCM. The annual risk of death in adults with HCM must be assessed individually and is around 1%; in children it is higher.
Clinical manifestations
Patients, especially in the non-obstructive form (HNCM), are often asymptomatic. If symptoms occur, they are usually not indicative (shortness of breath, angina pectoris , arrhythmia , dizziness , syncope , sudden death ).
Investigation methods - diagnostics
HCM must be differentiated from reactive myocardial hypertrophy caused by exercise ( athlete's heart ) or longstanding high blood pressure ( hypertensive heart ) as well as from disease of the aortic valve ( aortic valve stenosis ).
Medical history and physical examination
Besides little pioneering complaint information in the medical history (see above) falls on physical examination as part of the auscultation a systolic murmur (eg. Ten squats), which under stress or under a pressing maneuvers ( Valsalva maneuver ) increases or by the increase in the gradient in the left ventricular outflow tract becomes noticeable in the context of the increased load. This allows the HOCM to be differentiated relatively reliably from aortic valve stenosis or mitral valve insufficiency, since their noises become quieter during the Valsalva maneuver.
Technical investigation procedures
The ECG may show signs of left ventricular hypertrophy ( Sokolow-Lyon index ), Q waves and repolarization disorders , but these are unspecific.
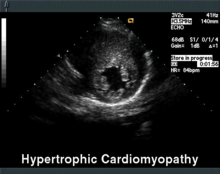
The echocardiography is the diagnostic tool of choice. In addition to septal hypertrophy (> 15 mm), a displaced mitral valve leaflet can often be seen here, which makes a movement towards the septum in the systole and additionally narrows the outflow tract (systolic anterior movement = SAM). Furthermore, the resting gradient in the left ventricular outflow tract (LVOT), i. H. a pressure jump between the left ventricle and the main artery , measured and the stiffening of the myocardium assessed.
In exercise echocardiography , the gradient in the LVOT can be determined before and after exercise, which provides an objective parameter for monitoring progress and therapy.
The MRI can also represent atypical (distribution) forms reliable and is therefore indicated for clinical suspicion and lack of proof of HOCM in echocardiography. She can detect spot-shaped scars in the hypertrophied myocardium, which are considered a risk factor for arrhythmias and sudden cardiac death . The acceleration of the flow in the outflow tract as well as the scarred changes after therapeutic septal embolization can also be clearly visualized using magnetic resonance imaging.
The cardiac catheter examination offers the possibility of a direct pressure measurement in the heart to determine the gradient in the LVOT, to determine the extent of the myocardial stiffening and to rule out other diseases. If a ventricular extrasystole is triggered during the catheter examination, the pressure gradient is multiplied and the systolic blood pressure does not increase during the post-extrasystolic beat (so-called Brockenbrough phenomenon). This is considered to be pathognomic for the HOCM.
In the laboratory there is the possibility of genetic diagnostics. However, this is very expensive and is therefore currently only carried out in the context of studies.
Another very simple method of monitoring progress and therapy is the 6-minute walk test .
Relatives screening
First-degree relatives should be screened for HCM. Children of sick parents have a 50 percent risk of also being carriers of the genetic defect. If genetic diagnosis is not possible (see above), children and adolescents between the ages of 12 and 18 should be examined annually by echocardiography and after the age of 18 every five years. Before the age of twelve, screening is only recommended for children who come from a high-risk family or who participate in competitive sports.
therapy
Conservative action
Physical exertion, depending on the severity of the disease, is permitted, but no competitive sport or sports with sudden maximum stress (soccer game) should be practiced. There is an increased risk of malignant cardiac arrhythmias.
In both forms of HCM, drugs are given that reduce the performance of the left ventricle (“less is more”). These include beta blockers or calcium antagonists of the verapamil type.
Antiarrhythmics are used for severe arrhythmias .
Drugs that strengthen the contractile strength of the heart muscle, such as digitalis or catecholamines , also increase the obstruction in HOCM and should therefore not be used. Likewise, pre- or afterload-lowering drugs such as nitro compounds , ACE inhibitors or AT1 antagonists lead to an increase in the gradient in the outflow tract and are also contraindicated .
Interventional measures
In the case of HOCM , the definitive treatment of the obstruction should be aimed for if the gradient in the LVOT is> 30 mmHg or if the gradient is after provocation. This can reduce the development of a diastolic compliance disorder . There are three therapy options available.
Catheter treatment of septal hypertrophy
The Transcoronare Ablation of septal hypertrophy (TASH), alcohol septal ablation (ASA), or percutaneous transluminal septal myocardial ablation (PTSMA) is the interventional procedure of choice for the treatment of obstruction of a HOCM. In this method, first carried out by Sigwart in June 1994 at the Royal Brompton Hospital in London, the first septal branch of the ramus interventricularis anterior (RIVA; LAD) is first found using a cardiac catheter and temporarily closed with a balloon. If the gradient in the left ventricular outflow tract then drops, pure alcohol is injected into this vessel through the balloon in order to trigger a circumscribed infarction in the area of the obstruction. This area will shrink and the obstruction will decrease over the following months.
The success rate is over 88%, the lethality under 1.2%. In about 10% of cases a permanent III ° AV block is caused, so that the implantation of a pacemaker is necessary . A second intervention is necessary in 15% of the cases. A very rare, potentially life-threatening side effect is the acute coronary no-flow phenomenon (ACNF).
A variant of catheter treatment that has not yet been established is ablation of the septal branch with the help of a cyanoacrylic .
Endocardial radiofrequency ablation of septal hypertrophy
Heart catheter-guided radiofrequency ablation for the treatment of cardiac arrhythmias has been around for some time. It was first used by Lawrenz in 2004 to treat HOCM . So far, the method has only been carried out at individual centers. It is used after unsuccessful alcohol ablation. The electrical energy is delivered by means of a cardiac catheter in the obstruction area on the right ventricular septum. As with alcohol ablation, this creates a scar to reduce the gradient in the left ventricular outflow tract. No conclusive data are yet available for an assessment of the long-term effectiveness.
Under the term radio frequency catheter ablation, the technique was first successfully used in two children (5 and 11 years old) in 2005.
Transaortic subvalvular myectomy
Today, surgery is the last option. In Morrow's heart surgery, which has been known for several decades, excess muscle tissue in the outflow tract of the left ventricle is removed through the aortic valve. The success rate and risks are similar to alcohol ablation, but the procedure is much more invasive.
Supportive measures
To protect against malignant cardiac arrhythmias, depending on the family history ( sudden cardiac death in first-degree relatives), implantation of a defibrillator (ICD) is often recommended. If malignant arrhythmias have already been documented, an ICD should always be implanted.
Two-chamber pacemaker therapy alone with a shortened atrial-chamber conduction time has not been found to be sufficiently effective. A decrease in the gradient in the LVOT can be demonstrated, but the subjective improvement in well-being is in the range of the placebo effect. As a supportive measure, implantation can be recommended in patients who also suffer from tachycardiac or bradycardic cardiac arrhythmias that require treatment or who are opposed to invasive therapy (see above).
forecast
HCM is a previously incurable disease, but if diagnosed early it is often easily treatable. Most people have a normal life expectancy with no limitations or the need for interventional measures. In some cases, however, there is a progressive course with the risk of serious complications.
Overall, the HOCM is usually the more serious form. The relative risk of HCM-related death (including sudden cardiac death) is 2.0. That is, the risk is twice as high with the HOCM compared to the HNCM. The relative risk of serious symptoms, according to NYHA Class III or IV , of death from heart failure, or of stroke is 4.4.
history
The disease was first described by Liouville and Hallopeau in 1869. It has been widely accepted as a clinical entity since its description by Sir Russell Brock in 1957 .
Literature and Sources
- Christian Mewis, Reimer Rissen, Ioakim Spyridopoulos (eds.): Kardiologie compact. Everything for ward and specialist examination . 2nd unchanged edition. Georg Thieme, Stuttgart a. a. 2006, ISBN 3-13-130742-0 .
- Gerd Herold : Internal Medicine. A lecture-oriented presentation. Taking into account the subject catalog for the medical examination. With ICD 10 key in the text and index . Herold, Cologne 2016.
- C. Prinz et al .: Diagnostics and therapy for hypertrophic cardiomyopathy . In: Dtsch Arztebl Int . tape 108 , no. 13 , April 2011, p. 209–215 , doi : 10.3238 / arztebl.2011.0209 .
- U. Raute-Kreinsen: Morphology of necrosis and repair after alcohol-induced transcoronary ablation of septal hypertrophy in HOCM . In: Pathology - Research & Practice . Elsevier Verlag, April 19, 2007 ( pathologie-bielefeld.de ).
- Shikhar Agarwal, E. Murat Tuzcu, Milind Y. Desai, Nicholas Smedira, Harry M. Lever, Bruce W. Lytle, Samir R. Kapadia: Updated Meta-Analysis of Septal Alcohol Ablation Versus Myectomy for Hypertrophic Cardiomyopathy . In: Journal of the American College of Cardiology . tape 55 , no. 8 , 2010, p. 823-834 , doi : 10.1016 / j.jacc.2009.09.047 .
Web links
- Cardiomyopathy, familial hypertrophic; CMH. In: Online Mendelian Inheritance in Man . (English).
- H. Kuhn: How to perform a safe and effective TASH. 2009, accessed October 27, 2009 .
Individual evidence
- ↑ Angelika Batzner, Hans-Joachim Schäfers, Konstantin V. Borisov, Hubert Seggewiß: Hypertrophic obstructive cardiomyopathy — the role of myectomy and percutaneous septal ablation in drug-refractory disease . In: Deutsches Aerzteblatt Online . January 25, 2019, ISSN 1866-0452 , doi : 10.3238 / arztebl.2019.0047 ( aerzteblatt.de [accessed on June 18, 2019]).
- ↑ Authors / Task Force members, PM Elliott, A Anastasakis, MA Borger, M Borggrefe, F Cecchi, P Charron, AA Hagege, A Lafont, G Limongelli, H Mahrholdt, WJ McKenna, J Mogensen, P Nihoyannopoulos, S Nistri, PG Pieper, B Pieske, C Rapezzi, FH Rutten, C Tillmanns, H Watkins: 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). In: European Heart Journal . tape 35 , no. 39 , 2014, p. 2733-2779 , doi : 10.1093 / eurheartj / ehu284 , PMID 25173338 .
- ↑ a b c d e f American College of Cardiology / European Society of Cardiology Clinical Expert: Consensus Document on Hypertrophic Cardiomyopathy. In: Journal of the American College of Cardiology . Volume 42, No. 9, 2003.
- ↑ F. Sedaghat-Hamedani, E. Kayvanpour, OF Tugrul, A. Lai, A. Amr, J. Haas, T. Proctor, P. Ehlermann, K. Jensen, Katus HA, B. Meder: Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: a meta-analysis on 7675 individuals . In: Clinical Research in Cardiology . August. doi : 10.1007 / s00392-017-1155-5 . PMID 28840316 .
- ↑ a b C. Hengstenberg: Genetics of familial hypertrophic cardiomyopathy . (PDF) In: Dtsch. Doctor bl. Volume 93, No. 9, 1996, pp. A-532 / B-430 / C-406.
- ↑ H. Kuhn, J. Mercier, E. Köhler, H. Frenzel, W. Hort, Franz Loogen : Differential diagnosis of hypertrophic cardiomyopathies: typical (subaortic) hypertrophic obstructive cardiomyopathy, atypical (midventricular) hypertrophic obstructive cardiomyopathy and hypertrophic non obstructive cardiomyopathy . In: Eur Heart J , Volume 4, Suppl F, 1983, pp. 93-104.
- ↑ Michael Schäfers et al .: Non-invasive cardiac imaging. In: ecomed medicine. 2008, ISBN 978-3-609-16282-9 .
- ↑ a b c Christian Mewis, Reimer Riessen, Ioakim Spyridopoulos (eds.): Cardiology compact - Everything for ward and specialist examination . 2nd Edition. Thieme, Stuttgart / New York 2006, ISBN 3-13-130742-0 , pp. 396-397 .
- ↑ a b L. Faber, Hubert Seghaben, FH Gietzen, H. Kuhn, P. Boekstegers, L. Neuhaus, L. Seipel, Dieter Horstkotte: Catheter-based septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: follow-up results of the TASH -registry of the German Cardiac Society. In: Zeitschrift für Kardiologie Volume 93, No. 1, January 2004, pp. 23–31; PMID 16049653 .
- ^ U. Sigwart: Non-surgical myocardial reduction for hypertrophic obstructive cardiomyopathy. In: The Lancet . Volume 346, No. 8969, July 22, 1995, pp. 211-214. PMID 7616800 .
- ^ H. Kuhn: Transcoronary ablation of septal hypertrophy (TASH): a 5-year experience. In: Z. Kardiol. Volume 89, No. 6, June 2000, pp. 559-564. PMID 10929441 .
- ↑ A. Oto, K. Aytemir, A. Deniz: New approach to septal ablation: glue (cyanoacrylate) septal ablation. In: Catheter Cardiovasc Interv. Volume 69, No. 7, June 1, 2007, pp. 1021-1025. PMID 17525960 .
- ↑ T. Lawrenz, H. Kuhn: Endocardial radiofrequency ablation of septal hypertrophy. A new catheter-based modality of gradient reduction in hypertrophic obstructive cardiomyopathy . In: Journal of Cardiology . tape 93 , no. 6 , June 2004, p. 493-499 , doi : 10.1007 / s00392-004-0097-x , PMID 15252744 .
- ↑ M. Emmel, N. Sreeram, JV deGiovanni, K. Brockmeier: Radiofrequency catheter septal ablation for hypertrophic obstructive cardiomyopathy in childhood . In: Journal of Cardiology . tape 94 , no. 10 , October 2005, p. 699-703 , doi : 10.1007 / s00392-005-0282-6 , PMID 16200487 .
- ↑ Claudia Strunk-Mueller, Frank H. Gietzen, Horst Kuhn: Pacemaker therapy for hypertrophic obstructive cardiomyopathy . In: Pacemaker Therapy and Electrophysiology . tape 15 , no. 1 , July 2004, ISSN 0938-7412 , p. i47-i53 , doi : 10.1007 / s00399-004-1107-4 .
- ↑ Bernard J. Gersh, Barry J. Maron, Robert O. Bonow, Joseph A. Dearani, Michael A. Fifer, Mark S. Link, Srihari S. Naidu, Rick A. Nishimura, Steve R. Ommen, Harry Rakowski, Christine E. Seidman, Jeffrey A. Towbin, James E. Udelson, Clyde W. Yancy: 2011 ACCF / AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy . In: American College of Cardiology / American Heart Association (Ed.): Circulation . tape 124 , no. 24 , December 13, 2011, pp. e783-e831 , doi : 10.1161 / CIR.0b013e318223e2bd .