Idebenone
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
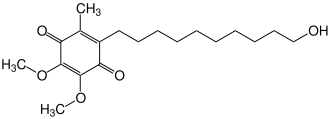
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Idebenone | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 19 H 30 O 5 | |||||||||||||||
| Brief description |
yellow powder |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 338.439 g · mol -1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
52-55 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Idebenone is an antioxidant , synthetically produced substance that is structurally and functionally similar to ubiquinone-10 . By inhibiting lipid peroxidation, it is able to protect cell membranes and mitochondria from damage by toxic forms of oxygen.
Idebenone is used, among other things, as a drug in the treatment of Leber's optic atrophy (LHON), for which it has been approved in the EU since October 2015. Idebenone is one of the psychoanaleptics and nootropics .
Pharmacological properties
Idebenone is an antioxidant that acts on the mitochondria . LHON patients have mutations in genes that encode mitochondrial functions. There are malfunctions in energy production, with toxic forms of oxygen ( free radicals ) that damage the retinal cells in the eye . Idebenone is said to help improve energy production by restoring the function of the mitochondria, thus preventing cell damage and loss of vision.
Idebenone can be used orally. The bioavailability is improved with simultaneous consumption with fatty food. Studies on pharmacodynamics and pharmacodynamics are described in the literature.
Medical use
Idebenone was originally developed by Takeda Pharmaceutical to treat Alzheimer's disease , but it was not shown to be sufficiently effective. In some European countries it is used to treat cognitive disorders.
The company Santhera Pharmaceuticals developed idebenone in other application areas . The drug is only available on prescription.
Leber optic atrophy
With the trade name: Raxone , idebenone was approved by the European Commission in September 2015 for the treatment of visual disturbances in adults and adolescents aged 12 and over with liver optic atrophy (LHON) . It is the first active substance approved for this indication in all 28 member states of the European Union (EU), Norway, Iceland and Liechtenstein.
LHON is a rare hereditary mitochondrial disease with a frequency of approximately 2 / 100,000 in Europe. Idebenone was already designated as an orphan drug (medicine for orphan diseases) in February 2007 . Hartmut Morck, Marburg, speaks in the Pharmazeutische Zeitung (PZ) in his assessment of a “leap innovation”.
In Germany, a study project in the form of a registry for patients was started in 2018 in order to gain important insights into the diagnosis, prognosis and cure chances of the rare disease LHON.
Friedreich's ataxia
The Friedreich's Ataxia (FA) is a progressive degenerative disease of the central nervous system . It is characterized by a genetic deficiency in the protein frataxin , which plays a role in the formation of the mitochondria. If there is a lack of frataxin, toxic oxygen radicals are formed, which damage nerve and heart muscle cells in particular . As a mnesis , idebenone was approved in Switzerland from 2004 to 2011 for the treatment of manifest non-dilated cardiomyopathy in FA patients, and in Canada from 2008 to 2013 under the name Catena . In 2008 the EMA rejected a European application for approval in this indication for the drug Sovrima .
Potential uses
Idebenone is also being investigated in other areas of indication, e.g. B. Duchenne muscular dystrophy (DMD), various other mitochondrial diseases, e.g. B. Cardiomyopathy , Friedreich's Ataxia , Kearns-Sayre Syndrome , Mitochondrial Encephalopathy, Lactic Acidosis & Stroke-like Episodes (MELAS) and / or thioredoxin-2 deficiency and ophthalmoplegia progressiva externa (CPEO).
A phase II study is being carried out in the USA for the indication of primary progressive MS (PP-MS) .
unwanted effects
Common side effects are a combined nasopharynx and cough. Diarrhea and back pain are also more common.
Clinical studies
- Leber optic atrophy (LHON)
- Idebenone was studied in the pivotal study RHODOS, which included 85 patients, in which it was compared with placebo for 24 weeks . The main focus for effectiveness was on improving visual acuity. Tests were mainly carried out with standard optotypes , which the patients were asked to read on an eye test chart as part of an eye test . By the end of the study, patients treated with idebenone were seeing an average of 3 to 6 more visual signs compared with those receiving placebo. Furthermore, some severely damaged patients were able to read at least one line in the eye test after treatment, which was also considered clinically relevant. 30% of patients treated with the drug (16 out of 53) achieved a clinically relevant improvement in vision in at least one eye, compared with 10% of patients (3 out of 29) in the placebo group. The results are supported by the RHODOS-OFU study.
- In previous studies, Raxone achieved a stabilization of visual acuity (Clinically Relevant Stabilization, CRS) in at least 1 in 2 patients with good residual vision in at least one eye. The drug also caused clinically relevant recovery (CRR) in 1 in 3 patients treated for up to 5 years after the onset of symptoms. A CRR was defined as an improvement from the off-chart area (ETDRS scale) to at least 5 letters on-chart (corresponds to 1 line) or an improvement of at least 10 letters on-chart (corresponds to 2 lines).
- Duchenne muscular dystrophy (DMD)
- The DELOS study examined the effectiveness of stabilizing lung function in patients with Duchenne muscular dystrophy at 17 centers in Europe and the USA. The 64 patients included with genetically confirmed DMD were between 10 and 18 years old.
Early benefit assessment (AMNOG)
Since 2011, newly approved drugs with new active ingredients in Germany have had to undergo an " early benefit assessment " by the Federal Joint Committee (G-BA) on the basis of Section 35a SGB V ( AMNOG ) . This also applies to idebenone.
The G-BA evaluated Raxone as part of the AMNOG procedure and assessed the extent of the additional benefit in LHON patients as “not quantifiable”. The severely limited evidence for LHON is due to the rarity of the disease. With a prevalence of LHON between 2.06 and 4.30 per 100,000 people, this disease occurs extremely rarely in Germany and requires a high level of effort to recruit patients for clinical studies. The European Medicines Agency (EMA) has granted approval under “exceptional circumstances”, recognizing the study situation. This means that due to the rarity of the disease, it was not possible to get complete information about this medicine. As part of risk management, the EMA will annually assess any new information that becomes available and update the package insert if necessary.
Like all newly launched drugs in Germany, Raxone has now gone through the AMNOG procedure. As part of this procedure, the National Association of Statutory Health Insurance Funds and Santhera agreed to a uniform reimbursement amount for Raxone . As part of the negotiations with the Statutory Health Insurance Funds Association for refund was for prescribing Raxone a practice specialty agreed.
Finished medicinal products
Raxone (EU), Mnesis (IT), Amizal (PT)
Other uses
In the US, idebenone is marketed as a dietary supplement . Idebenone is a synthetic substance that is not found in food. In 2003 the FDA took the view that idebenone did not meet the definition of a dietary supplement or dietary ingredient . Idebenone was also sold as a dietary supplement in other European countries.
The substance is used in cosmetic products to prevent skin aging.
literature
- B. Leo-Kottler, B. Wissinger: Liver optic neuropathy. In: The ophthalmologist. 108, 2011, pp. 1179-1194, doi: 10.1007 / s00347-011-2482-y .
- BRAIN: Barboni P, et al .: Idebenone treatment in patients with OPA1-mutant dominant optic atrophy . In: Brain - A Journal of Neurology . February 1, 2013, doi : 10.1093 / brain / aws280 .
- Front Neurol: Barboni P, et al .: Medical Management of Hereditary Optic Neuropathies . In: frontiers in Neurology . July 31, 2014, doi : 10.3389 / fneur.2014.00141 .
- BRAIN: Holzerova E, et al .: Human thioredoxin 2 deficiency impairs mitochondrial redox homeostasis and causes early-onset neurodegeneration . In: Brain - A Journal of Neurology . February 1, 2016, doi : 10.1093 / brain / awv350 .
- EMBO: Koopman W JH, et al .: Mitochondrial disorders in children: toward development of small ‐ molecule treatment strategies . In: EMBO - Molecular Medicine . March 8, 2016, doi : 10.15252 / emmm.201506131 .
Individual evidence
- ↑ Entry on HYDROXYDECYL UBIQUINONE in the CosIng database of the EU Commission, accessed on August 4, 2020.
- ↑ a b c d e Data sheet Idebenone, ≥98% (HPLC) from Sigma-Aldrich , accessed on November 21, 2015 ( PDF ).
- ↑ a b c d Summary of the EPAR for the public of the EMA (German), accessed on November 18, 2015.
- ^ Roman H Haefeli, et al .: NQO1-Dependent Redox Cycling of Idebenone: Effects on Cellular Redox Potential and Energy Levels . In: PLOS ONE . tape 6 , no. 3 , March 2011, p. e17963 , doi : 10.1371 / journal.pone.0017963 .
- ↑ Michael Erb, et al .: Features of Idebenone and Related Short chain Quinones did Rescue ATP levels under Conditions of Impaired Mitochondrial Complex I . In: PLOS ONE . tape 7 , no. 4 , April 2012, p. e36153 , doi : 10.1371 / journal.pone.0036153 .
- ↑ Summary of the European public assessment report (EPAR) for Raxone , the EMA (English), accessed on November 18, 2015.
- ↑ Santhera launches Raxone® in the first EU country , PM Santhera from October 1, 2015, accessed on November 18, 2015.
- ↑ Santhera receives European marketing authorization for Raxone® in liver hereditary optic neuropathy (LHON) , PM Santhera from September 9, 2015, accessed on November 18, 2015.
- ^ Meta-analysis of the prevalence of Leber hereditary optic neuropathy mtDNA mutations in Europe , Eur J Ophthalmol 2012; 22 (3): 461-465, doi: 10.5301 / ejo.5000055 .
- ↑ A handful of new active ingredients , PZ 45/2015, accessed on February 3, 2016.
- ↑ Leber Hereditary Optic Neuropathy - Pro Retina initiates first German patient registry ( Memento from September 5, 2018 in the Internet Archive ); PM PRO RETINA Germany e. V. of September 5, 2018, accessed on September 5, 2018.
- ↑ Mnesis® (idebenone) - no extension of the temporary approval ; accessed on August 4, 2020.
- ↑ Idebenone - Voluntary withdrawal from the Canadian market , WHO Pharmaceuticals Newsletter No. 2 2013.
- ↑ Sovrima on the website of the European Medicines Agency; accessed on August 4, 2020.
- ↑ Idebenon at DMD ( Memento from June 27, 2018 in the Internet Archive ), website of the manufacturer, accessed on June 27, 2018.
- ↑ a b Guidelines for Mitochondrial Diseases , DGN website, accessed on June 27, 2018.
- ↑ Kearns-Sayre Syndrome , on Symptomat.de, accessed June 27, 2018.
- ↑ Study of Idebenone in the Treatment of Mitochondrial Encephalopathy Lactic Acidosis & Stroke-like Episodes (MELAS) , ClinicalTrials.gov, accessed June 27, 2018.
- ↑ Mitochondrial disorders in children: toward development of small ‐ molecule treatment strategies , EMBO Molecular Medicine, Volume8, Issue4, April 2016, pp. 311–327.
- ↑ Human thioredoxin 2 deficiency impairs mitochondrial redox homeostasis and causes early-onset neurodegeneration , Brain, Volume 139, Issue 2, February 1, 2016, pp. 346–354.
- ↑ Primary Progressive Multiple Sclerosis on the manufacturer's website, accessed November 18, 2015.
- ↑ Thomas Klopstock, et al .: A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy . In: Brain . tape 134 , no. 9 , September 2011, p. 2677-2686 , doi : 10.1093 / brain / awr170 .
- ↑ Thomas Klopstock, et al .: Persistence of the treatment effect of idebenone in Leber's hereditary optic neuropathy . In: Brain . tape 136 , no. 2 , February 2013, p. e230 – e230 , doi : 10.1093 / brain / aws279 .
- ↑ Klopstock T et al., Brain, 134: 2677-2686, 2011; Metz G et al., "EUNOS", Ljubljana, 2015.
- ↑ Hasham et al., “ARVO,” Seattle, 2016.
- ↑ Gunnar M Buyse, et al .: Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomized placebo-controlled phase 3 trial . In: The Lancet . tape 385 , no. 9979 , May 2015, p. 1748–1757 , doi : 10.1016 / S0140-6736 (15) 60025-3 .
- ↑ Resolutions on the benefit assessment of drugs with new active ingredients according to Section 35a SGB V - Idebenone , G-BA resolution of March 17, 2016, accessed on September 7, 2017.
- ↑ Summary of the risk management plan (RMP) for Raxone (idebenone) EMA's risk management plan .
- ^ FDA memorandum, March 2, 2003
- ↑ D. McDaniel, B. Neudecker, J. Dinardo, J. Lewis, H. Maibach: Clinical efficacy assessment in photodamaged skin of 0.5% and 1.0% idebenone. J Cosmet Dermatol. 4 (3): 167-73 (2005). PMID 17129261 . doi: 10.1111 / j.1473-2165.2005.00305.x
