Isoprocarb
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Isoprocarb | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 11 H 15 NO 2 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 193.24 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.62 g cm −3 |
||||||||||||||||||
| Melting point |
93-96 ° C |
||||||||||||||||||
| boiling point |
128-129 ° C |
||||||||||||||||||
| solubility |
easily in water (265 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Isoprocarb is a synthetic insecticide from the active ingredient group of carbamates . It was introduced by Bayer in 1970 .
Extraction and presentation
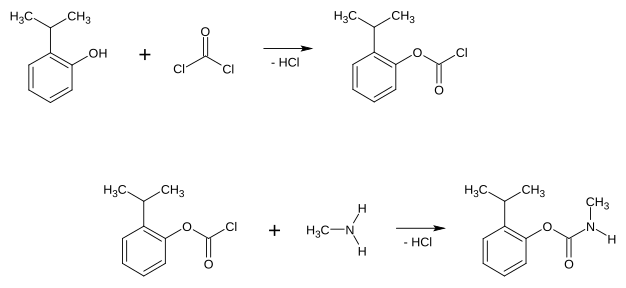
Isoprocarb can be obtained by reacting 2-isopropylphenol with phosgene and methylamine .
Mode of action
Isoprocarb works in the same way as all carbamate insecticides. It inhibits the enzyme acetylcholine esterase in the synapses of the nervous system . This causes the nerves to stop transmitting stimuli, which can lead to paralysis and even respiratory failure and ultimately death.
Areas of application
Isoprocarb can be used against a wide range of insects. For example, it is used in fruit and vegetable cultivation against aphids and bugs , cicadas , beetles and psyllids .
toxicology
Isoprocarb is classified as a Class II toxin (moderately dangerous) by the World Health Organization . Isoprocarb poisoning can cause symptoms such as muscle weakness, dizziness, fatigue, nausea and headache, as well as disorders of the central nervous system. In studies with chickens could be found that Isoprocarb adverse changes in blood counts caused and hepatotoxic is.
Isoprocarb is toxic to bees and aquatic organisms and is classified as hazardous to the environment. The compound is not very persistent in the environment with a half-life of 3 to 20 days.
Analytics
Liquid chromatographic methods can be used for reliable detection and quantification of Isoprocarb . Identification can be carried out after the chromatographic separation with a mass spectrometer .
Admission
Isoprocarb is not approved in the European Union . The maximum residue level in all foods is 0.01 mg / kg. It is approved in Cambodia , Vietnam and the Philippines .
Individual evidence
- ↑ a b c d e Entry on Isoprocarb. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2018.
- ↑ a b c d e f g h i Entry on Isoprocarb in the GESTIS substance database of the IFA , accessed on June 19, 2018 (JavaScript required)
- ↑ Entry on Isoprocarb in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on June 19, 2018. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . Noyes Publications, 1996, ISBN 978-0-8155-1401-5 , pp. 85 ( limited preview in Google Book search).
- ↑ International Program on Chemical Safety., Inter-Organization Program for the Sound Management of Chemicals., World Health Organization .: WHO recommended classification of pesticides by hazard and guidelines to classification 2009. World Health Organization, Geneva 2010, ISBN 92-4154796- 0 .
- ↑ a b c Paranjape, Kalyani .: The pesticide encyclopedia . CABI, Wallingford, Oxfordshire UK 2014, ISBN 978-1-78064-014-3 .
- ↑ MF Rahman, MKJ Siddiqui, M. Mahboob, M. Mustafa: Haematological and hepatotoxic effects of isoprocarb in chicken . In: Journal of Applied Toxicology . tape 10 , no. 3 , June 1990, ISSN 0260-437X , p. 187-192 , doi : 10.1002 / jat.2550100308 .
- ↑ Derick Lucas: Optimizing Sample Preparation for LC / MS / MS of Pesticide Residues in Herbal Teas. (PDF) In: Agilent Technologies, Inc. December 17, 2013, accessed June 28, 2018 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Isoprocarb in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on May 19, 2018.