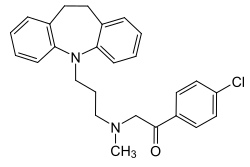
Lofepramine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Non-proprietary name | Lofepramine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 26 H 27 ClN 2 O | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| Drug information | ||||||||||
| ATC code | ||||||||||
| Drug class | ||||||||||
| properties | ||||||||||
| Molar mass | 418.96 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
104-106 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Lofepramine is a chemical from the dibenzazepine class that is used as a drug to treat depression . It belongs to the group of active substances called tricyclic antidepressants .
The drug was patented in 1970 and 1972 by the Swedish stock corporation Leo ( Leo Läkemedel AB ) and was on the market in Germany under the trade name Gamonil ® from Merck .
Presentation and extraction
The synthesis starts from the drug desipramine from, the secondary amine structure with 2-bromo-4'-chloroacetophenone alkylated is.
Clinical information
The mean daily dose of lofepramine is between 70 and 210 mg.
Pharmacological properties
Lofepramine is structurally a derivative of imipramine , which is metabolized to the active metabolite desipramine . The drug is used in the form of its hydrochloride .
The substance inhibits the reuptake of norepinephrine from the synaptic cleft to a much greater extent than that of serotonin . Lofepramine also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
literature
- Harald Schmidt (Ed.), Founded by Claus-Jürgen Estler: Pharmacology and Toxicology. 6th edition Schattauer, Stuttgart a. New York 2007. p. 241 u. 245.
Individual evidence
- ^ Römpp Lexikon Chemie. 10 ed. Thieme, Stuttgart a. New York 1996-1999. P. 2441.
- ↑ a b Lofepramine hydrochloride data sheet from Sigma-Aldrich , accessed on February 1, 2013 ( PDF ).
- ↑ a b Entry on lofepramine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ^ A. Kleemann , J. Engel, B. Kutscher, D. Reichert: Pharmaceutical Substances - Synthesis, Patents, Applications , 4th edition (2001) Thieme-Verlag Stuttgart, ISBN 978-1-58890-031-9 .
- ↑ Ernst Mutschler: drug effects. 7th edition. Wissenschaftliche Verlagsgesellschaft, Stuttgart 1996. p. 156.
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .
