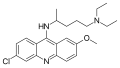
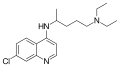
Mepacrine
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| 1: 1 mixture of ( R ) -form (top) and ( S ) -form (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Mepacrine | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 23 H 30 ClN 3 O | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 399.96 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
248-250 ° C (Di hydrochloride dihydrate) |
|||||||||||||||||||||
| pK s value |
10.3 |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Mepacrine is a drug that has historically been used for therapy and malaria prophylaxis . Mepacrine is still occasionally used today to treat Giardia infections.
development
The effect of acridine - derivatives as antiprotozoic had Paul Ehrlich in 1912 in the treatment of trypanosomiasis found in animal experiments. After the war, a large number of derivatives were tested for their effects on protozoal and bacterial infections in various laboratories. Mepacrine was discovered around 1930 by Walter Kikuth at IG Farbenindustrie in Elberfeld (Bayer) in a screening program of around 12,000 substances as a promising antimalarial agent. It was synthesized by the chemists Fritz Mietzsch and Hans Mauss at Bayer. Mepacrine was marketed under various trade names . It became known under the name Atebrin ® or (in the USA) Quinacrine (Atabrine ® ).
Mechanism of action
The mechanism of action of mepacrine is unclear; binding to DNA through intercalation may play a role. Mepacrine also acts as a FIASMA (functional inhibitor of acid sphingomyelinase ).
Historical meaning
The development of synthetic anti-malarials was of military importance, as it made it independent of the overseas supply of cinchona bark for quinine production . During the Second World War , mepacrine played no role on the German side, as even better drugs such as Sontochin , which is closely related to chloroquine , had been developed in the meantime .
On the part of the Allies, the production of mepacrine was considered to be important to the war effort, accordingly Imperial Chemical Industries in Great Britain and, from 1941 in the USA, the Winthrop company were commissioned to manufacture it. Under the name Quinacrine, mepacrine was the most important antimalarial drug used by the Allies in Southeast Asia. Improved remedies like chloroquine were only available after the end of the war. According to Walter Sneader, the war production of quinacrine and penicillin at the time was the cornerstone for the United States to become the largest pharmaceutical manufacturer in the world in the post-war period.
Todays use
Mepacrine is not approved as a medicinal product. In different countries it can be used for protozoan infections in human and veterinary medicine in individual cases with a special permit from the authorities.
literature
- W. Kikuth: For the further development of synthetically produced antimalarials. I. On the chemotherapeutic effect of atebrine , Deutsche Medizinische Wochenschrift 58 ( 1932 ) 530-531. doi : 10.1055 / s-0028-1122899
- W. Sneader: Drug Discovery. A history. Wiley 2005 ISBN 0-471-89980-1 .
Individual evidence
- ↑ a b Entry on mepacrine in the DrugBank of the University of Alberta .
- ↑ a b Quinacrine dihydrochloride data sheet from Sigma-Aldrich , accessed on April 9, 2011 ( PDF ).
- ↑ Entry on mepacrine in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P: Identification of novel functional inhibitors of acid sphingomyelinase . In: PLoS ONE . 6, No. 8, 2011, p. E23852. doi : 10.1371 / journal.pone.0023852 .