Chloroacetaldehyde
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chloroacetaldehyde | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 3 ClO | |||||||||||||||
| Brief description |
highly volatile, colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 78,50 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.19 g cm −3 |
|||||||||||||||
| Melting point |
−16.3 ° C |
|||||||||||||||
| boiling point |
85-86 ° C |
|||||||||||||||
| Vapor pressure |
139 m bar (25 ° C) |
|||||||||||||||
| solubility |
easily in water with hydrate formation (443 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
Switzerland: 1 ml m −3 or 3 mg m −3 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
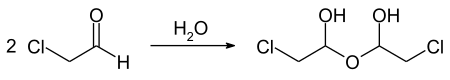
Chloracetaldehyde is a chemical compound from the group of aldehydes . It is next to the Dichloroacetaldehyde and trichloroacetaldehyde one of three possible chlorinated acetaldehyde. The compound forms a relatively stable aldehyde hydrate ClCH 2 CH (OH) 2 .
Presentation and extraction
The Wacker-Hoechst process for the production of acetaldehyde from ethene produces chloroacetaldehyde as a by-product. Anhydrous chloroacetaldehyde can be obtained by the oxidation of α-chlorohydrin using periodate . The aldehyde can also be obtained in good yield by the pyrolysis of chloroethylene carbonate .
A specific synthesis by the α-chlorination of acetaldehyde is also known.
properties
Chloracetaldehyde is a colorless, pungent smelling liquid that boils at 85 ° C under normal pressure . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in Torr, T in ° C) with A = 7.26359, B = 1338.8586 and C = 220.432 in the temperature range from −7 to 107 ° C. The critical values are 282 ° C for the critical temperature, 53.7 bar for the critical pressure , 0.201 l · mol −1 for the critical volume and 0.3905 g · cm −3 for the critical density . The compound is readily soluble in water with hydrate formation and in common organic solvents. In water, a dimeric acetal, also known as a hemihydrate, chemically 1,1'-dihydroxy-2,2'-dichlorodiethyl ether is formed. This compound forms colorless crystals that melt between 43 and 50 ° C and appear to boil at 84 ° C, breaking down into the starting aldehyde and water.
The solubility in water is determined by the content of the hemihydrate compound.
Solubility of the hemihydrate in water temperature in ° C 1 10 20th 30th 40 concentration in% 13.35 22.2 44.3 62.7 81.5
The hemihydrate compound can be cyclized in the presence of concentrated sulfuric acid to give trichloroparaldehyde (2,4,6-trichloromethyl-1,3,5-trioxane), the trimer of chloroacetaldehyde. The trimer forms colorless crystals that melt at 88–89 ° C.
The tetramer tetrachlorometaldehyde (2,4,6,8-tetrakis (chloromethyl) -1,3,5,7-tetroxocane) can be obtained from the hemihydrate compound by azeotropic dehydration . The compound forms colorless crystals that melt at 65–67 ° C or evaporate at a pressure of 1.3 Pa at 127–130 ° C.
The anhydrous compound tends to form trimers, tetramers and polymers with a polyoxymethylene structure when stored at room temperature.
Chloracetaldehyde forms flammable vapor-air mixtures at high temperatures. With a flash point of 70 ° C, the substance is considered flame-retardant. The explosion range is between 3.9 vol.% As the lower explosion limit (LEL) and 9.0 vol.% As the upper explosion limit (UEL).
Toxicology and Occupational Safety
Contact with the compound in liquid or vapor form can cause excessive irritation or even burns to the eyes, respiratory tract and skin. The formation of pulmonary edema cannot be ruled out. The LD 50 value (rat, oral) is 75 mg kg −1 .
use
Chloracetaldehyde is used as a versatile starter or intermediate in organic synthesis. In the synthesis of heterocycles such. B. for pyrroles , furans , thiophenes , imidazoles , oxazolines , thiazolines , thiazoles or indoles , the compound is an important starting substance. Chloracetaldehyde is used as a raw material for the production of pharmaceuticals , insecticides , fungicides , disinfectants , dyes , hardeners for epoxy resins and antistatic agents .
Individual evidence
- ↑ a b c d e f g h i Entry on chloroacetaldehyde in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e Entry on chloroacetaldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on April 17, 2015.
- ↑ a b c e-EROS Encyclopedia of Reagents for Organic Synthesis , 1999-2013, John Wiley and Sons, Inc., entry for Chloroacetaldehyde, accessed November 4, 2015 .
- ↑ Entry on chloroacetaldehyde in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Chloroacetaldehyde solution, produced by Wacker Chemie AG, Burghausen, Germany, ≥45.0% in H2O at Sigma-Aldrich , accessed on April 11, 2015 ( PDF ).
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 107-20-0 or chloroacetaldehyde ), accessed on November 2, 2015.
- ↑ a b c d e f g h i j k Jira, R .; Kopp, E .; McKusick, BC; Röderer, G .; Bosch, A .; Fleischmann, G .: Chloroacetaldehydes in Ullmann's Encyclopedia of Industrial Chemistry , 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 / 14356007.a06_527.pub2 .
- ↑ Hatch, LF; Alexander, HE: Preparation of Chloroacetaldehyde Hydrate in J. Am. Chem. Soc. 67 (1945) 688-688, doi : 10.1021 / ja01220a504 .
- ↑ Gross, H .: About α-halogen ethers. XVI. Monochloroacetaldehyde or derivatives of glycolaldehyde and glyoxal from α-halogen ethers in J. Prakt. Chem. 21 (1963) 99-102, doi : 10.1002 / prac.19630210115 .
- ^ Yaws, CL: The Yaws Handbook of Vapor Pressure: Antoine Coefficients , 2nd edition, Elesevier 2015, ISBN 978-0-12-802999-2 .
- ^ Carl L. Yaws, Prasad K. Narasimhan: Critical Properties and Acentric Factor - Organic Compounds. In: Carl L. Yaws (Ed.): Thermophysical Properties of Chemicals and Hydrocarbons. Elsevier, 2009, ISBN 978-0-8155-1596-8 .
- ↑ Natterer, K .: About Monochloraldehyd in Monatsh. Chem. 3 (1882) 442-464, doi : 10.1007 / BF01516819 .
- ↑ Kopp, E .; Smidt, J .: Reactions with chloroacetaldehyde and 2,4-dichloro-crotonaldehyde in Justus Liebigs Ann. Chem. 693 (1966) 117-127, doi : 10.1002 / jlac.19666930110 .
- ↑ Quijano, ML; Nogueras, M .; Sanchez, A .; Alvarez de Cienfuegos, G .; Melgarejo, M .: Synthesis, anticancer and antimicrobiological activities of pyrrolo [2,3-d] pyrimidines in J. Heterocyclic Chem. 27 (1990) 1079-1083, doi : 10.1002 / jhet.5570270449 .
- ↑ Bisagni, E .; Rivalle, C .: in Bull. Soc. Chim. Fr. 1974, the 519th
- ↑ Padwa, A .; Gasdaska, JR: Generation of sulfur ylides by the desilylation of α-trimethylsilylbenzyl sulfonium salts in Tetrahedron 44 (1988) 4147-4156, doi : 10.1016 / S0040-4020 (01) 86662-X .
- ↑ Matsumoto, M .; Wanatabe, N .: in Heterocycles 22 (1984) 2313.
- ↑ Hirota, K .; Shirahashi, M .; Senda, S .; Yogo, M .: Pyrimidines. 65. Synthesis of 6-substituted thieno [2,3-d] pyrimidine-2,4 (1H, 3H) -diones in J. Heterocyclic Chem. 27 (1990) 717-721, doi : 10.1002 / jhet.5570270345 .
- ↑ Aldvogel, E .: Synthesis of 2-substituted and 2,3-disubstituted alkyl- and aryl-thiophenes and 2,3-fused thiophene derivatives from ketones as C 2 building blocks and carbonodithioic acid-O-ethyl-S- (2 -oxoethyl) ester as a C 2 S building block in Helv. Chim. Acta 75 (1992) 907-912, doi : 10.1002 / hlca.19920750325 .
- ↑ Kluge, AF: Synthesis of an imidazo [1,2-c] pyrimidine analog of a thiamine antagonist coccidiostat and a comparison of several methods for the preparation of imidazo [1,2-c] pyrimidines in J. Heterocyclic Chem. 15 ( 1978) 119-121, doi : 10.1002 / jhet.5570150125 .
- ↑ Senga, K .; Robins, RK; O'Brien, DE: Synthesis of certain imidazo [2,1-f] pyrazolo [3,4-d] pyrimidines in J. Heterocyclic Chem. 12 (1975) 1043-1044, doi : 10.1002 / jhet.5570120547 .
- ↑ Maya Weber, Jürgen Jakob, Jürgen Martens : Synthesis and reactivity of 3-oxazolines. In: Liebig's annals of chemistry . 1992, pp. 1-6, doi: 10.1002 / jlac.199219920102 .
- ↑ Jürgen Martens, Heribert Offermanns , Paul Scherberich: Simple synthesis of racemic cysteine. In: Angewandte Chemie . 93, 1981, pp. 680-683, doi: 10.1002 / anie.19810930808 .
- ↑ Begtrup, M .; Hansen, LBL: New Methods for the Introduction of Substituents into Thiazoles in Acta Chem. Scand. 46 (1992) 372-383, doi : 10.3891 / acta.chem.scand.46-0372 .
- ^ Brandsma, L .; De Jong, RLP; VerKruijsse, HD: An Efficient Synthesis of 1,3-Thiazole in Synthesis 1985, 948-949, doi : 10.1055 / s-1985-31396 .
- ↑ Wender, PA; White, AW: Methodology for the facile and regio-controlled synthesis of indoles in Tetrahedron 39 (1983) 3767-3776, doi : 10.1016 / S0040-4020 (01) 88618-X .