Butanal
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
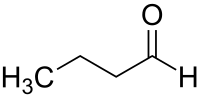
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Butanal | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 8 O | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 72.11 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.80 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−97 ° C |
|||||||||||||||
| boiling point |
75 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.3843 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
20 ml m −3 , 64 mg m −3 |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−239.2 kJ mol −1 |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Butanal (according to IUPAC nomenclature: n -butanal , also known as butyraldehyde ) is an organic-chemical compound from the group of aldehydes . In addition to the linear n-butanal, there is also the isomeric compound iso-butanal (isobutyraldehyde).
Extraction and presentation
N- Butanal is produced on an industrial scale only by the hydroformylation of propene at temperatures of 90-125 ° C. and pressures of 10-60 bar. The catalyst systems used are usually rhodium and sulfonated triarylphosphines in aqueous solution .
The byproduct iso butanal ( 2-methylpropanal ). Butanal can also be obtained by gentle oxidation of the corresponding alcohol 1-butanol .
properties
Physical Properties
Butanal is a colorless, pungent smelling, volatile, highly flammable liquid that boils at 75 ° C at normal pressure . According to Antoine, the vapor pressure function results from log 10 (P) = A− (B / (T + C)) (P in bar, T in K) with A = 3.59112, B = 952.851 and C = −82.569 in the temperature range from 303.86 to 347.18 K.
Compilation of the most important thermodynamic properties property Type Value [unit] Remarks Standard enthalpy of formation Δ f H 0 liquid
Δ f H 0 gas−245.4 kJ mol −1
−211.8 kJ mol −1as a liquid
as a gasEnthalpy of combustion Δ c H 0 liquid −2477.1 kJ mol −1 Heat capacity c p 164.7 J mol −1 K −1 (25 ° C)
2.28 J g −1 K −1 (25 ° C).as liquid
as liquidCritical temperature T c 537.2 K Critical pressure p c 43.20 bar Critical density ρ c 3.88 mol·l −1 Critical volume V c 0.258 l mol −1 Enthalpy of fusion Δ F H 10.773 kJ mol −1 at the melting point Enthalpy of evaporation Δ V H 32.9 kJ mol −1 at normal pressure boiling point
The solubility of water in butanal changes only slightly with increasing temperature. In contrast, the solubility of butanal in water decreases with increasing temperature.
| Solubilities in the butanal - water system | ||||||||||||
| temperature | in ° C | 0 | 10 | 20th | 30th | 40 | 50 | |||||
| Solubility of water in butanal | in% | 3.2 | 2.8 | 2.6 | 2.4 | 2.4 | 2.4 | |||||
| Solubility of butanal in water | in% | 9.8 | 8.6 | 7.6 | 6.8 | 6.1 | ||||||
With a water content of 8.8% by mass of the compound forms a boiling at 68 ° C azeotrope . Further azeotropically boiling mixtures are formed with ethanol and n- hexane .
| Azeotropes with various solvents | ||||||||||||
| solvent | water | Ethanol | n-hexane | |||||||||
| Content of butanal | in% | 91.2 | 59.4 | 26th | ||||||||
| boiling point | in ° C | 68 | 70.7 | 60 | ||||||||
Safety-related parameters
Butanal forms highly flammable vapor-air mixtures. The compound has a flash point of −11 ° C. The explosion range is between 1.7% by volume (51 g / m 3 ) as the lower explosion limit (LEL) and 12.5% by volume (375 g / m 3 ) as the upper explosion limit (UEL). The maximum explosion pressure is 7.5 bar. The limit gap width was determined to be 0.92 mm. This results in an assignment to explosion group IIA. The ignition temperature is 190 ° C. The substance therefore falls into temperature class T4.
Chemical properties
Butanal polymerizes when heated and under the influence of acids and alkali. It reacts with oxidizing agents , strong acids, strong bases and amines .
use
Butanal is required for the production of vulcanization accelerators , synthetic resins and plasticizers . It is the basis of synthetic tanning and fragrances . In addition, n- butanol is produced on an industrial scale from n- butanal by catalytic hydrogenation . Furthermore, it can be converted into butanoic acid (butyric acid) by oxidation .
safety instructions
The substance can be absorbed into the body by inhalation of the vapors and by ingestion. It irritates the eyes , skin and airways. The vapor is heavier than air and can spread across the floor.
The formation of explosive peroxides is possible.
Individual evidence
- ↑ a b c d e f g h i j k l m n o p q r Entry on butyraldehyde in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ a b c d Entry on butyraldehyde. In: Römpp Online . Georg Thieme Verlag, accessed on August 22, 2017.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-72.
- ↑ Entry on butyraldehyde in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-26.
- ^ Kessen Günther, Cornils Boy, Hibbel Josef, Bach Hans-Wilhelm, Gick Wilhelm, Wiebus Ernst, Zgorzelski Wolfgang: METHOD FOR THE PRODUCTION OF 2-ETHYLHEXANOL. In: http://www.freepatentsonline.com . Ruhrchemie AG, May 3, 1987, accessed on February 3, 2019 .
- ↑ M. Seprakova, J. Paulech, J. Dykyj: Steam pressure of butyraldehydes. In: Chem. Zvesti. 13, 1959, pp. 313-316.
- ↑ a b K. B. Wiberg, LS Crocker, KM Morgan: Thermochemical studies of carbonyl compounds. 5. Enthalpies of reduction of carbonyl groups. In: J. Am. Chem. Soc. 113, 1991, pp. 3447-3450, doi: 10.1021 / ja00009a033 .
- ↑ GR Nicholson: 478. The heats of combustion of butanal and heptanal. In: J. Chem. Soc. 1960, pp. 2377-2378.
- ↑ a b c V. G. Vasil'ev, BV Lebedev: Thermodynamics of butanal in the temperature range 0-330K. In: Zh. Obshch. Khim. 59, 1989, pp. 2415-2420.
- ↑ a b A. S. Teja, DJ Rosenthal: The Critical Pressures and Temperatures of Twelve Substances Using A Low Residence Time Flow Apparatus. In: AIChE Symp. Ser. 86, 279, 1990, pp. 133-137.
- ↑ MJ Anselme, AS Teja: The critical properties of rapidly reacting substances. In: AIChE Symp. Ser. 86, 279, 1990, pp. 128-132.
- ↑ a b c d e D. K. Raff: Butanals. In: Ullmann's Encyclopedia of Technical Chemistry . Wiley-VCH Verlag, Weinheim 2013. doi : 10.1002 / 14356007.a04_447.pub2 .
- ↑ JG Wojtasinski: Measurement of Total Pressures for Determining Liquid-Vapor Equilibrium Relations of the Binary System Isobutyraldehyde n-Butyraldehyde. In: J. Chem. Eng. Data . 8, 1963, pp. 381-385, doi: 10.1021 / je60018a028 .
- ^ A b c d E. Brandes, W. Möller: Safety-related parameters - Volume 1: Flammable liquids and gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Entry on butanols. In: Römpp Online . Georg Thieme Verlag, accessed on February 4, 2019.


