n -Octyl-β- D -1-thioglucopyranoside
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | n -Octyl-β- D -1-thioglucopyranoside | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 14 H 28 O 5 S | |||||||||||||||||||||
| Brief description |
white, crystalline solid |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 308.43 g mol −1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
|
|||||||||||||||||||||
| solubility | ||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
n -Octyl-β- D -1-thioglucopyranoside (Octylthioglucoside, OTG) is a thioglucoside and a mild nonionic surfactant for the solubilization of proteins , especially membrane proteins .
Occurrence and representation
n -Alkyl thioglycosides of the n- octyl-β- D -thioglucopyranoside type have no naturally occurring representatives. In contrast, mustard oil glycosides are widely used as natural S-glycosides .
The synthesis of n- octyl-β- D -thioglucopyranoside starts from D -glucose (I), which is peracetylated with acetic anhydride and concentrated sulfuric acid to give α- D -glucopyranose pentaacetate ( pentaacetylglucose ) (II).
Pentaacetylglucose reacts with hydrogen bromide to form 2,3,4,6-tetra-O-acetyl-α- D -glucopyranosyl bromide (acetobromoglucose) (III), which with thiourea in acetone almost quantitatively forms the isothiuronium salt 2,3,4,6-tetra- O -acetyl-β- D -glucopyranosyl-1-isothiuronium bromide (IV) forms.
The nucleophilic thiolate anion formed after neutralization of the salt and reduction with sodium sulfite to the thiol in the alkaline also reacts almost quantitatively with 1-bromooctane to form n- octyl-2,3,4,6-tetra- O- acetyl-1-thio-β - D -glucopyranoside (peracetylated octylthioglucoside) (V), from which the alkaline deacetylation using sodium hydroxide in methanol quantitatively produces the target product n- octyl-1-thio-β- D -glucopyranoside (VI) in a total yield of approx. 80%.
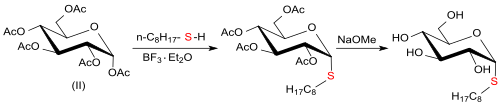
In the trichloroacetimidate method according to Richard R. Schmidt , the peracetylated O- (α- D -glucopyransyl) trichloroacetimidate with 1-octanethiol under boron trifluoride etherate catalysis with inversion (chemistry) (after deacetylation) exclusively the n -octyl- 1-thio-β- D -glucopyranoside, while the perbenzylated O- (α- D- glucopyransyl) trichloroacetimidate is converted exclusively into the n- octyl-1-thio-α- D -glucopyranoside with retention (chemistry) (after debenzylation) .
By reacting D- glucose with 1-octanethiol with Olah's reagent (70% hydrogen fluoride HF in pyridine ), however, the anomeric mixture n- octyl-1-thio-α, β- D -glucopyranoside is formed in 95% yield, which is 44% Contains α-anomer and 56% β-anomer.
The pure α-octylthioglucoside is accessible by reacting pentaacetyl-β- D -glucose (from D- glucose, acetic anhydride and sodium acetate ) in organic solvents at elevated temperatures with 1-octanethiol and boron trifluoride etherate and subsequent deacetylation.
properties
n -Octyl-β- D -1-thioglucopyranoside is a colorless, odorless, hygroscopic, crystalline solid that dissolves easily in water and lower alcohols. Compared to the O -glucoside n -octyl-β- D -glucopyranoside, which was introduced earlier as a detergent for biochemical applications , the analogous S -glucoside OTG appears particularly suitable due to its higher stability, especially against degradation by β-glucosidases.
| Comparison of S- octyl glucoside with O- glucoside | ||||||||||||
| properties | Critical Micellar Concentration ( CMC) | Solubilizing power | Dialysability | Chem. Stability | β-glucosidase stability | Transparency at 280 nm | Denaturation tendency | Chem. Analytics | ||||
| n -Octyl-β- D -thiogluco-pyranoside | 9 mM (+) | ++ | + | + | + | + | + | + | ||||
| n -Octyl-β- D -gluco-pyranoside | 23-25mM + | ++ | ++ | (-) | - | + | + | + | ||||
++ very good + good (+) partially good (-) weak - bad
The cost advantage for octylthioglucoside put forward in publications from the 1980s apparently no longer exists because of the more recently developed efficient enzymatic synthesis routes for O- octyl glucoside (directly from D- glucose, 1-octanol using β-glucosidase).
The α-anomeric octylthioglucoside shows liquid-crystalline properties with the formation of a smectic phase A.
Applications
Nonionic detergents solubilize membrane proteins gently and while (largely) maintaining their physiological function through interaction with the hydrophobic membrane areas embedded in the lipid bilayers of cell membranes. Above the so-called critical micelle concentration CMC [OTG: 9 mM, or 0.2772% (w / v)], mixed micelles are formed from membrane proteins and surfactant molecules, with OTG concentrations of 1.1 - 1.2% (w / v) v) were used for the solubilization of membrane proteins from E. coli. No denaturation of the membrane proteins was found after solubilization with octylthioglucoside.
For the analysis of the biological activity of membrane proteins it is often necessary to reconstitute the proteins in the lipid bilayers of liposomes . The solution of the solubilized protein is subjected to dialysis or ion exchange chromatography in the presence of phospholipids or membrane lipid mixtures to remove the surfactant . Under standard conditions, 95% of the OTG can be removed from a 43 mM surfactant solution after 6 hours.
Octylthioglucoside (15 mM) is clearly superior to its O- analogue octylglucoside (OT) in solubilizing and stabilizing against thermal and light-induced denaturation of the light-driven proton pump bacteriorhodopsin from the biomembrane of halobacteria .
Individual evidence
- ↑ a b c d data sheet Octyl-β-D-1-thioglucopyranoside ≥ 98.0% from Sigma-Aldrich , accessed on February 25, 2017 ( PDF ).
- ↑ a b Data sheet n-Octyl-beta-D-thioglucopyranoside, 98.0% from AlfaAesar, accessed on February 25, 2017 ( PDF )(JavaScript required) .
- ↑ a b c T. Tsuchiya, S. Saito: Use of n-Octyl-β-D-thioglucoside, a new nonionic detergent, for solubilization and reconstitution of membrane proteins . In: J. Biochem. tape 96 , no. 5 , 1984, pp. 1593–1597 , doi : 10.1093 / oxfordjournals.jbchem.a134989 .
- ↑ a b c S. Saito, T. Tsuchiya: Characteristics of n-octyl-β-D-thioglucopyranoside, a new nonionic detergent useful for membrane biochemistry . In: Biochem. J. Band 222 , 1984, pp. 829-832 , doi : 10.1042 / bj 2220829 .
- ^ A b S. Saito, T. Tsuchiya: Synthesis of Alkyl-β-D-thioglucopyranoside, a series of new nonionic detergents . In: Chem. Pharm. Bull. Volume 33 , no. 2 , 1985, pp. 503-508 , doi : 10.1248 / cbp.33.503 .
- ↑ CE Redemann, C. Niemann: Acetobromoglucose [2,3,4,6-Tetraacetyl-α-d-glucopyranosyl bromide] In: Organic Syntheses . 22, 1942, p. 1, doi : 10.15227 / orgsyn.022.0001 ; Coll. Vol. 3, 1955, p. 11 ( PDF ).
- ^ RU Lemieux: Methods in Carbohydrate Chemistry, Vol. 2, Reactions of Carbohydrates . Ed .: RL Whistler, ML Wolfrom, JN BeMiller. Academic Press Inc., New York 1963, pp. 221-222 .
- ^ RR Schmidt, M. Stumpp: Glycosylimidate. 8. Synthesis of 1-thio-glycosides . In: Liebigs Ann. Chem. Band 1983 , no. 7 , 1983, pp. 1249-1256 , doi : 10.1002 / jlac.198319830717 .
- ↑ GA Olah, JG Shih, GKS Prakash: Fluorine-containing reagents in organic synthesis . In: J. Fluorine Chem. Volume 33 , no. 1-4 , 1986, pp. 377-396 , doi : 10.1016 / S0022-1139 (00) 85282-3 .
- ↑ Patent US5118804 : Process for preparing alkyl-1-thioglycosides and alkyl-glycosides, anomer mixtures thereof. Applied July 31, 1990 , published June 2, 1992 , Applicant: Beghin-Say, SA, Inventor: J. Defaye, A. Gadelle, C. Pedersen.
- ↑ Patent EP1041080B1 : Process for the preparation of pentaacetyl-beta-D-glucopyranose. Applied on February 18, 2000 , published on October 4, 2000 , Applicants: Nisshin Flour Milling Co., Ltd., Inventors: M. Tsuji, H. Yamazaki.
- ↑ a b H.A. van Doren, R. van der Geest: Synthesis and liquid crystalline properties of the n-alkyl-1-thio-α-D-glucopyranosides, a new homologous series of carbohydrate mesogens . In: Carbohydrate Research . tape 194 , 1989, pp. 71-77 , doi : 10.1016 / 0008-6215 (89) 85007-4 .
- ↑ A. Ducret, J.-F. Carrière, M. Trani, R. Lortie: Enzymatic synthesis of octyl glucoside by almond β-gluconidase in organic media . In: Can. J. Chem. Volume 80 , no. 6 , 2002, pp. 653-656 , doi : 10.1139 / v02-081 .
- ↑ OTG, 1-S-Octyl-β-D-Thioglucopyranoside (Octylthioglucose) 28351. (PDF; 44 kB) (No longer available online.) Pierce Chemical Co., January 1998, archived from the original on March 12, 2017 ; accessed on February 26, 2017 (English). Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ A. Asada, M. Sonoyama: Solubilization and structural stability of bacteriorhodopsin with a mild nonionic detergent, n-octyl-β-thioglucoside . In: Biosc. Biotechnol. Biochem. tape 75 , no. 2 , 2011, p. 376–378 , doi : 10.1271 / bbb.100726 .