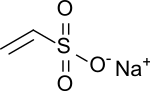
Sodium vinyl sulfonate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium vinyl sulfonate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 3 NaO 3 S | |||||||||||||||
| Brief description |
colorless solid, yellow-brown aqueous solutions are commercially available |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 130.10 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.18-1.22 g · cm −3 |
|||||||||||||||
| Vapor pressure |
18.16 mmHg at 21 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium vinyl sulfonate , short SVS (of English Sodium sulfonates called), is a sodium salt of vinylsulfonic unsaturated the simplest representative alkene . Its bifunctional nature with an olefinic double bond and a reactive sulfonic acid group makes SVS a useful organic intermediate and versatile monomer building block for homo- and copolymers .
Extraction and presentation
Sodium vinyl sulfonate is formed during the alkaline hydrolysis of carbyl sulfate :
During the reaction, it is obtained as a dilute aqueous solution which can be concentrated by spray drying and converted into solid sodium vinyl sulfonate.
properties
The yellow-brown aqueous sodium vinyl sulfonate solution formed during the hydrolysis of carbyl sulfate is adjusted to 25 to 35% by weight of SVS and a pH of 8 to 12.5. SVS tends to react with atmospheric oxygen and to polymerize, but is stable for at least six months when stabilized (e.g. with 100 ppm MEHQ or 20ppm Cupferron) and properly stored (below 25 ° C and in the dark).
use
Sodium vinyl sulfonate solution is used as a brightener in nickel and chrome electroplating baths. 1- (2-sulfoethyl) -pyridinium betaine from the class of sulfobetaines , which is obtained by reacting SVS with pyridine in an acidic medium, is a further development as a top-quality brightener in the deposition of nickel . Sodium vinyl sulfonate reacts with a variety of nucleophilic agents to form the corresponding secondary products, such as. B. with phenols, mercaptans, amines to taurine derivatives, etc., and especially with alcohols to form isethionates . This sulfethylation reaction can also be carried out as a polymer-analogous reaction with polymers containing hydroxyl groups, such as. B. use cellulose or cellulose ethers to introduce sulfoethyl groups. The reaction of SVS with hydroxyalkylamines gives, after acidification, hydroxyalkylaminosulfonic acids, which are important buffer substances, such as. B. TES represent.
Sodium vinyl sulfonate is a versatile monomer from which homopolymers and copolymers with vinyl derivatives, such as. B. vinyl acetate and with acrylic acid derivatives such. B. acrylic acid esters , acrylamide , acrylonitrile , etc. can be produced. Because of their good temperature stability and tolerance to calcium ions, copolymers with acrylamide are interesting as drilling aids in tertiary oil production ( enhanced oil recovery ). Terpolymers with acrylic acid and vinyl acetate are suitable as dispersants in industrial water treatment.
Furthermore, graft copolymers are accessible, e.g. B. with polyacrylonitrile to improve the dyeability, color fastness, antistatic properties, abrasion resistance, etc. of the fibers made therefrom.
Further application examples for SVS and its copolymers are known.
Individual evidence
- ↑ a b c Entry for CAS no. 3039-83-6 in the GESTIS substance database of the IFA , accessed on August 8, 2012(JavaScript required) .
- ↑ Technical information and safety data sheets from BASF AG for Golpanol R VS and WeylChem Frankfurt GmbH for sodium vinyl sulfonic acid 30%
- ↑ a b N-SVS-25, Kowa American Corp., Specifications Sodium Vinylsulfonate ( Memento of the original from February 7, 2016 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF; 117 kB).
- ↑ a b Entry on Sodium Vinylsulfonate (25% in Water, approx. 2.3mol / L) at TCI Europe, accessed on August 10, 2012.
- ↑ a b c data sheet from Kessler Chemical ( Memento of the original dated December 3, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , accessed August 8, 2012.
- ↑ DS Breslow, RR Hough, J. Amer. Chem. Soc., 1957, 79 (18), 5000-5002; U.S. Patent US 3,872,165, inventor: W. Schenk et al., Applicant: BASF AG, issued March 18, 1975; German Offenlegungsschrift DE-OS 30 47 028 A1, inventors: H. Distler, R. Widder, applicant: BASF AG, published on July 15, 1982.
- ↑ US patent US 3,805,870, inventor: W. Schenk, D. Stockburger, applicant: BASF AG, issued April 23, 1974.
- ^ "Practical electroplating technology", Eugen G. Lenze Verlag, Saulgau, 4th edition 1984, pp. 268-271.
- ^ European patent specification EP 1 054 866 B1, inventor: E. Kappes et al., Applicant: BASF AG, published on November 29, 2000.
- ↑ H. Distler, Angew. Chem., 77 (7), 291-311 (1965).
- ↑ PCT patent WO 1993009125 A1, inventor: M. Weuthen, applicant: Henkel KGaA, published on May 13, 1993.
- ↑ German Offenlegungsschrift DE-OS 42 43 281 A1, inventor: R. Kiesewetter et al., Applicant: Wolff Walsrode AG, published on June 23, 1994.
- ↑ German Patent DE 4203529 A1, inventor R. Kiesewetter et al, Applicant: Wolff Walsrode AG, published on 12 September 1993. U.S. Patent US 5,278,305, inventor: R. Kniewske et al., Applicant: Wolff Walsrode AG, issued January 11, 1994.
- ↑ U.S. Patent US 4,481,150, inventor: K. Ishii et al., Assignee: Nippon Paint, issued November 6, 1984 and U.S. Patent US 4,582,651, inventor: K. Ishii et al., Assignee: Nippon Paint, issued on April 15, 1986.
- ↑ J. Cao et al., J. Polm. Res. (2011), 18: 171-178
- ↑ U.S. Patent US 4,604,211, inventor: JF Kneller, JR Hurlock, assignee: Nalco Chemical, issued August 5, 1986.

