Orellanin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Orellanin | ||||||||||||||||||
| other names |
3,3 ′, 4,4′-tetrahydroxy-2,2′-bipyridine- N , N ′ -dioxide ( IUPAC ) |
||||||||||||||||||
| Molecular formula | C 10 H 8 N 2 O 6 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 252.18 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
Decomposes at 270 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Orellanin is a nephrotoxin (kidney toxin ) found in some veils ( mushrooms ) with an unusual bipyridine structure. It belongs to the heterocyclic compounds and to the phenols .
history
In 1952, a minor epidemic of poisoning occurred in Poland , affecting 102 people, 11 of whom died. It was discovered that all those affected had consumed mushrooms of the species Cortinarius orellanus , which until then had been considered edible. The Polish scientist Grzymala then compiled the first systematic report on poisoning by Cortinarius orellanus in the years from 1953 to 1962. He listed 136 cases with 25 deaths. Grzymala isolated the toxin from the fungus and consequently named it orellanin . The substance was just as toxic to animals as it was to humans; the organs affected were in all cases the kidneys . In 1972 and 1981, other Cortinarius species ( C. speciosissimus , C. splendens ) were also found to be poisonous, with a total of 240 affected.
Occurrence
Orellanin occurs in some fungi from the genus Cortinarius , e.g. B. in the orange-foxed rocky head ( Cortinarius orellanus ) and in the pointed-hunched rocky head ( C. rubellus ).
According to studies from 2003, it is found in the fruiting bodies of the pointed hunched head as orellanin diglucoside and is only converted into orellanin in the stomach.
properties
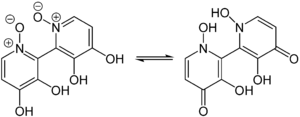
The colorless, dark blue fluorescent fungus poison with the empirical formula C 10 H 8 O 6 N 2 is stable up to about 150 ° C and then begins to convert into orellin and the non-toxic, yellow orellin and oxygen. Above 267 ° C there is sometimes explosive decomposition. The decomposition can also take place through UV light . Orellanin is insoluble in water and non-polar organic solvents, but it dissolves well in dimethyl sulfoxide , methanol , pyridine and trifluoroacetic acid . Orellin is an amphoteric substance due to the presence of hydroxyl groups and nitrogen atoms , which also has complex-forming properties.
Poisonous effect
In cats and guinea pigs, LD 50 values of 4.9 to 8.3 mg kg −1 were determined, and in mice, depending on the source, LD 50 (oral) between 9 and 90 mg kg −1 . The effect of the poison is based on the inhibition of alkaline phosphatase and the synthesis of proteins , RNA and DNA . Above all, orellanin is highly toxic to the kidneys. The first symptoms of Orellanus Syndrome are thirst, kidney pain, drying up of urine production, and headache. The symptoms of intoxication only appear after a latency period of 3 to 14 days. Since the decomposition is very slow when heated, the orellanin is not destroyed by boiling the mushrooms.
proof
The detection of orellanin according to Pöder and Moser succeeds with very little moistened fungal substance, which after adding a few drops of iron (III) chloride solution immediately indicates the presence of the toxin by means of a purple discoloration.
Individual evidence
- ↑ Entry on orellanin. In: Römpp Online . Georg Thieme Verlag, accessed on November 24, 2014.
- ↑ a b c d D. G. Spoerke, BH Rumack: Handbook of Mushroom Poisoning: Diagnosis and Treatment. CRC Press, 1994, ISBN 0-8493-0194-7 , pp. 250-255.
- ↑ There is not yet a harmonized classification for this substance . A label of [No public or meaningful name is available] in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 5, 2019, is reproduced from a self-classification by the distributor .
- ↑ a b H. Prast, ER Werner, W. Pfaller, M. Moser: Toxic properties of the mushroom Cortinarius orellanus. I. Chemical characterization of the main toxin of Cortinarius orellanus (Fries) and Cortinarius speciosissimus (Kuhn & Romagn) and acute toxicity in mice . In: Arch. Toxicol. tape 62 , 1988, PMID 3190463 .
- ↑ a b J. Holmdahl: Mushroom poisoning: Cortinarius speciosissimus nephrotoxicity . Göteborg University, 2001 ( docstoc.com [PDF]).
- ↑ a b c d J. J. Kleber, Th. Zilker: Orellanus Syndrome. Toxinfo mushroom database, 2000.
- ↑ P. Spiteller, M. Spiteller, W. Steglich: On the occurrence of the mushroom poison orellanin as diglucoside and studies on its biosynthesis. In: Angew. Chem. 115, 2003, pp. 2971-2974; Angew. Chem. Int. Ed. 42, 2003, pp. 2864-2867.
- ↑ a b Gifte.de: Orellanin .
- ↑ René Flammer, Thomas Flammer: Mykologische Emergency Diagnostics. 3rd, revised. Edition. 2009, OCLC 837382723 .


