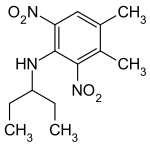
Pendimethalin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Pendimethalin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 13 H 19 N 3 O 4 | ||||||||||||||||||
| Brief description |
orange-yellow odorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 281.31 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.85 g cm −3 |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
330 ° C |
||||||||||||||||||
| Vapor pressure |
1.94 10 −3 Pa (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Pendimethalin is an active ingredient for crop protection and a chemical compound from the group of dinitroanilines .
Extraction and presentation
Pendimethalin can be obtained from o-xylene by reacting with nitric acid. The reaction produces the two possible regioisomers of nitroxylene. The reaction of 4-nitro-m-xylene with 3-pentanone and hydrogen gives the secondary amine , which is nitrated again with nitric acid to give pendimethalin .
Newer syntheses start with 4-nitro-o-xylene .
properties
Pendimethalin occurs in two polymorphic crystal forms. Polymorph I forms orange-colored crystals and forms a triclinic crystal lattice with space group P 1 (space group no. 1) . The polymorph II forms light yellow crystals and exists in a monoclinic crystal lattice with the space group P 2 1 / c (no. 14) . Polymorph I is the thermodynamically stable form at room temperature. The compound is practically insoluble in water. It is stable in the pH range from 4 to 9.
use
Pendimethalin is used as an active ingredient in crop protection products. It is made by American Cyanamid and used e.g. B. sold under the trade names Stomp , Sitradol and Prowl . It is used as a selective herbicide against most annual grasses and broad-leaved weeds in winter crops, corn, potatoes, rice, cotton, soybeans, tobacco, peanuts, and sunflowers. It is used both pre-emergence and early post-emergence. The effect is based on the inhibition of the formation of microtubules and the cell division as well as the disruption of the alignment of microfibrils.
In the USA in 2009 over 4000 t were consumed.
In a study that examined the pollution of tree bark by pesticides in Germany, the connection could be found in 87% of the site samples. It was the most commonly identified pesticide in the study. In the past, long-distance transport from neighboring fields that were kilometers away meant that organically grown fennel was contaminated with residues of pendimethalin and prosulfocarb , which made it impossible to use it in baby food. Residues of the two compounds were also found in kale.
Admission
In many EU countries, including Germany and Austria, as well as Switzerland, plant protection products containing this active ingredient are approved.
Individual evidence
- ↑ a b c d e f g Entry on Pendimethalin in the GESTIS substance database of the IFA , accessed on February 7, 2017(JavaScript required) .
- ↑ a b c d e EU: Review report for the active substance pendimethalin - Finalized in the Standing Committee on the Food Chain and Animal Health at its meeting on November 13, 2002 in view of the inclusion of pendimethalin in Annex I of Directive 91/414 / EEC (PDF; 332 kB)
- ↑ a b c Stockton, GW; Godfrey, R .; Hitchcock, P .; Mendelsohn, R .; Mowery, PC; Rajan, S .; Walker, AF Crystal polymorphism in pendimethalin herbicide is driven by electronic delocalization and changes in intramolecular hydrogen bonding. A crystallographic, spectroscopic and computational study in J. Chem. Soc. Perkin Trans. 2, 1998, 2061-2071.
- ↑ Entry on N- (1-ethylpropyl) -2,6-dinitro-3,4-xylidine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can use the harmonized classification and labeling expand .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 , pp. 872 ( limited preview in Google Book search).
- ↑ China Papers: Green Synthesis of Pendimethalin ( Memento of March 4, 2016 in the Internet Archive )
- ^ M. Bahadir, H. Parlar, Michael Spiteller: Springer Umweltlexikon . Springer, 2000, ISBN 978-3-540-63561-1 , pp. 871 ( limited preview in Google Book search).
- ↑ Entry Pendimethalin at Extoxnet.
- ^ Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals, Volume 2 . Royal Society of Chemistry, 1999, ISBN 978-0-85404-499-3 , pp. 270 ( limited preview in Google Book search).
- ↑ Dipl.-Biol. Frieder Hofmann, forest manager Ulrich Schlechtriemen, Dr. Maren Kruse-Plaß, Dr. Werner Wosniok: Biomonitoring of pesticide pollution in the air by means of air quality bark monitoring and multi-analysis for> 500 active ingredients including glyphosate. February 10, 2019, accessed March 8, 2019 .
- ↑ Bioland: Bioland calls for a ban on the herbicides Pendimethalin and Prosulfocarb. Retrieved March 8, 2019 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Pendimethalin in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 13, 2016.



