Phoxime
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
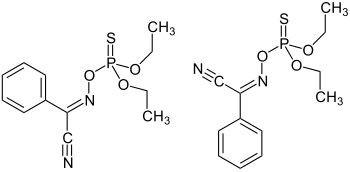
|
||||||||||||||||
| Mixture of ( E ) -Phoxime (left) and ( Z ) -Phoxime (right) | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Phoxime | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 15 N 2 O 3 PS | |||||||||||||||
| Brief description |
yellow to reddish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 298.30 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.18 g cm −3 |
|||||||||||||||
| Melting point |
6.1 ° C |
|||||||||||||||
| boiling point |
Decomposes on heating |
|||||||||||||||
| Vapor pressure |
2.1 m Pa at 20 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Phoxim is an organic compound from the group of thiophosphoric acid esters , which is mainly used in veterinary medicine and wood protection as an insecticide and acaricide . It is in the form of a yellow to reddish liquid.
history
Phoxim was launched by Bayer in 1968 under the name Baythion .
Working principle
The effect as contact poison, food poison and respiratory poison is based on the inhibition of acetylcholinesterase after metabolic desulfurization to the phosphoric acid ester . The two stereoisomers of phoxime have different levels of activity as an insecticide. The cis isomer - measured as a KT50 value - acts faster on organisms such as houseflies or the larvae of the common mosquito and the owl butterfly Mythimna separata than the trans isomer , both as a contact poison and as a food poison . The stereoselectivity is different depending on the species; it is particularly pronounced in mosquito larvae.
Admission
The approval of plant protection products containing Phoxim was withdrawn in Europe on June 21, 2007. In Switzerland, too, no pesticides containing this active ingredient are permitted.
proof
Acute poisoning can be detected by determination of cholinesterase or gas chromatography . The maximum permissible amounts in tea and cereals as well as in other plant-based foods are between 0.1 mg · kg −1 and 0.05 mg · kg −1 according to the Maximum Residue Quantity Ordinance .
Trade names
- Baythion
- Sebacil
- Valexon
See also
- Phoximmethyl , C 10 H 11 N 2 O 3 PS
- Chlorophoxime , C 12 H 14 ClN 2 O 3 PS
- Dichlorvos
- Parathion
literature
- Report of the Committee for veterinary medicinal products on Phoxim (PDF file; English, 40 kB)
Web links
- Joint FAO / WHO Expert Committee on Food Additives (JECFA), Monograph for Phoxim
- WHO / FAO Data Sheet on Pesticides (PDS) for Phoxim ( Memento of July 2, 2014 in the Internet Archive )
Individual evidence
- ↑ a b c d e f g Entry on Phoxim in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ Entry on Phoxim at Vetpharm, accessed on August 5, 2012.
- ↑ Entry on Phoxim in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet Phoxim, analytical standard at Sigma-Aldrich , accessed on January 27, 2019 ( PDF ).
- ↑ a b c Entry on Phoxim. In: Römpp Online . Georg Thieme Verlag, accessed on March 5, 2011.
- ↑ Wang Wenli et al .: Steroselecivity in Insecticidal Activities of Geometric Isomers of Phoxim . In: Pesticides. 1998, 37, 9, pp. 20-21 ( abstract ).
- ↑ DECISION OF THE COMMISSION of June 21, 2007 on the non-inclusion of certain active substances in Annex I of Directive 91/414 / EEC (PDF) .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Phoxim in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 27, 2016.
- ↑ External identifiers or database links for Phoximmethyl : CAS number: 14816-16-1, PubChem : 6506394 , ChemSpider : 5005731 , Wikidata : Q27286361 .
- ↑ External identifiers or database links for chlorophoxime : CAS number: 14816-20-7, EC number: 238-888-9, ECHA InfoCard: 100.035.338 , PubChem : 9570291 , ChemSpider : 7844758 , Wikidata : Q27155754 .


