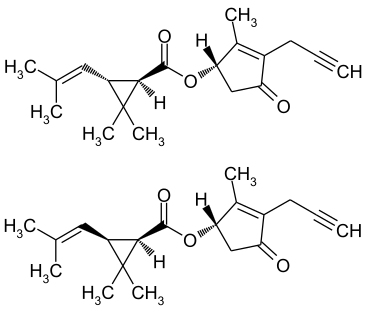
Prallethrin
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Prallethrin | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 19 H 24 O 3 | ||||||||||||||||||
| Brief description |
yellow to brown liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 300.40 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.03 g cm −3 |
||||||||||||||||||
| Melting point |
−25 ° C |
||||||||||||||||||
| boiling point |
313.5 ° C |
||||||||||||||||||
| Vapor pressure |
<1.33 · 10 −5 Pa at 23.1 ° C |
||||||||||||||||||
| solubility |
almost insoluble in water (8 mg l −1 at 25 ° C, pH 5.5-5.6) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Prallethrin is a synthetic insecticide from the group of pyrethroids . It was first described in 1961 and brought onto the market in 1988 by Sumitomo Chemical .
Presentation and structure
The chloride of chrysanthemum acid can be reacted with 4-hydroxy-3-methyl-2- (2-propargyl) -cyclopent-2-en-1-one to form prallethrin.
Prallethrin is very similar to allethrin . It only differs in the saturation of the propargylic side chain. The E-ISO name Prallethrin and the CAS number refer to a racemic mixture of eight stereoisomers . The WHO specification of the substance specifies a certain, deviating, isomer ratio, the trans - cis ratio being around 4: 1.
Properties and mode of action
Prallethrin is a yellowish to brown, oily liquid. It has a slightly phenolic odor and is almost insoluble in water. The substance is unstable to UV light , so that high persistence in the environment cannot be assumed.
Prallethrin, like other pyrethroids, acts as a contact poison on insects. It irreversibly blocks the sodium channels of the nervous system so that they remain constantly open. This results in paralysis, which leads to respiratory failure and ultimately death. Prallethrin can make insects unable to move within a very short time after contact ( knock-down effect).
use
Prallethrin is used against mosquitoes , house flies and cockroaches in the home. It is also used in veterinary medicine to treat pets in some countries .
Prallethrin is commercially available in the form of vaporizer plates for electric vaporizers or as a spray .
toxicity
Prallethrin is moderately toxic if taken orally or if inhaled as an aerosol . The oral LD 50 was determined to be 640 mg / kg of body weight for male rats and 460 mg / kg of body weight for female rats. The lethal concentration in the breath (LC 50 ) is 855 in male and 658 mg / m³ (exposure time 4 hours) in female rats. In animal experiments, prallethrin is not irritating to the skin , not sensitizing and minimally irritating to the eyes. It is not mutagenic , there is no evidence of a carcinogenic or reproductive toxicity. Prallethrin is very toxic to aquatic organisms and bees, but only slightly toxic to birds.
safety instructions
Prallethrin must not be stored in the vicinity of flammable materials and food or feed.
Individual evidence
- ↑ a b c d e f g h i WHO specification and evaluation for public health pesticides - Prallethrin . (PDF) World Health Organization Geneva, November 2004, accessed June 25, 2019 .
- ↑ a b c d e Entry on Prallethrin in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Entry on Prallethrin in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on June 25, 2019.
- ↑ Entry on 2-methyl-4-oxo-3- (prop-2-ynyl) cyclopent-2-en-1-yl 2,2-dimethyl-3- (2-methylprop-1-enyl) cyclopropanecarboxylate in the Classification and Labeling inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ PG Piquett, WA Gersdorff: The Relative Effectiveness of Two Synthetic Pyrethroids More Toxic to House Flies than Pyrethrins in Kerosene Sprays . In: Journal of Economic Entomology . tape 54 , no. 6 , December 1, 1961, ISSN 0022-0493 , p. 1250–1252 , doi : 10.1093 / jee / 54.6.1250 ( oup.com [accessed June 26, 2019]).
- ↑ Matsuo, Noritada., Mori, Tatsuya., Campbell, PJ: Pyrethroids: from chrysanthemum to modern industrial insecticide . Springer, Berlin 2012, ISBN 978-3-642-27346-9 ( limited preview in Google book search [accessed June 25, 2019]).
- ↑ Thomas A. Unger: Pesticide synthesis handbook . Noyes Publications, 1996, ISBN 0-8155-1401-8 , pp. 955 ( limited preview in Google Book Search).
- ↑ a b Entry on Prallethrin in the Hazardous Substances Data Bank , accessed June 26, 2019.
- ↑ Terry R. Roberts, David H. Hutson, Philip W. Lee, Peter H. Nicholls, Jack R. Plimmer: Metabolic Pathways of Agrochemicals . Part 2: Insecticides and Fungicides . Royal Society of Chemistry, 1999, ISBN 0-85404-499-X , pp. 690 ( limited preview in Google Book search).

