Pulvinon
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
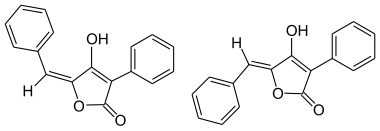
|
||||||||||
| ( E ) shape (left) and ( Z ) shape (right) | ||||||||||
| General | ||||||||||
| Surname | Pulvinon | |||||||||
| other names |
5-phenylmethylene-4-hydroxy-3-phenylfuran-2 (5 H ) -one ( IUPAC ) |
|||||||||
| Molecular formula | C 17 H 12 O 3 | |||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 322.33 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
243-247 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
The pulvinone is an organic chemical compound , to the lactones , esters , enols and to the group of pulvinic - dyes counts. Although pulvinone is not a natural product , various naturally occurring hydroxylated derivatives are known which are produced by fungi such as the golden boletus ( Boletus elegans , also known as Suillus grevillei ), or by molds such as Aspergillus Terreus . Compared to vulpic acid and pulvic acid, pulvinone has a low toxicity .
History and occurrence
There are many different dyes in mushrooms such as the bolete relatives and lichens , which are either derivatives of pulvic acid ( e.g. gomphidic acid ) or composed of several pulvic acid units (example of two units: badiones ). In 1831 the pulvic acid methyl ester vulpinic acid was discovered by the French pharmacist and chemist Antoine Bebert while studying lichen ( Cetraria vulpina ), but it was not until 1860 that Franz Möller and Adolph Strecker examined and described it in more detail. In an attempt to clarify the structure of vulpic acid, Adolf Spiegel found in 1880 that vulpic acid could be saponified into a diacid. He called the resulting diacid pulvic acid . In 1894 the constitution of pulvic acid was clarified by Jacob Volhard , who was able to synthesize pulvic acid by basic hydrolysis of a corresponding dinitrile. He also received small amounts of a by-product, which a year later was presented separately by Ludwig Claisen and Th. Ewan and characterized as 5-benzylidene-4-hydroxy-3-phenylfuran-2 (5 H ) -one.
Claisen and Ewan described it as the lactone on which pulvic acid is based : This is how the name Pulvinon came about shortly afterwards.
Biological importance
Interestingly, the name pulvinone only became a collective term a century after the discovery of the original pulvinone , namely when the first natural hydroxylated derivative was isolated by Edwards and Gill in 1973. It was a trihydroxylated pulvinone, which was found as a yellow pigment in the European mushroom golden boletus ( Boletus elegans , also known as Suillus grevillei ). In the same year 1973, Seto and his colleagues found pulvinone in cultures of the mold Aspergillus terreus . In order to distinguish these from the natural product isolated from Suillus grevillei , these compounds were called aspulvinones and named alphabetically according to the chromatographic elution sequence. (The most unpolar aspulvinone thus became aspulvinone A, the closest eluting to aspulvinone B, etc.).
Like many other yellow coloring agents in mushrooms and lichens, pulvinone is derived from pulvic acid. The pulvinon structural motif is thus represented in many natural substances. All derivatives of Pulvinsäuremonomers as pulvinic itself and about the Gomphidsäure that vulpinic that Aspulvinone , and derivatives of such Pulvinsäuredimers. B. the Badione , Norbadione or the pulvinone dimer such as aurantricholone contain this motif. As a rule, all naturally occurring pulvinone derivatives are ( Z ) -configured .
biosynthesis
In mushrooms, biosynthesis takes place via aromatic amino acids such as phenylalanine and tyrosine ; From this, after oxide amination to arylpyruvic acid, dimerization, ring cleavage and decarboxylation, the pulvinon skeleton is finally formed.

Chemical properties
Pulvinone is a lactone , i.e. an intramolecular ester of trans-1,4-diphenyl-2,3-dihydroxy-1,3-butadiene-1-carboxylic acid and is formed from this by splitting off water:
Pharmacological activity
- Rehse et al demonstrated that members of the Pulvinone family show anticoagulant activity in rats.
- In the early 1980s, ICI and Smith Kline & French patented many derivatives of vulpinic acid because of their anti-inflammatory , antipyretic and analgesic properties. However, like many of its derivatives, vulpinic acid is a cell poison . Since pulvinones showed lower cytotoxicity compared to the corresponding vulpic acid derivatives, the anti-inflammatory potential of more than 100 pulvinones was investigated by Organon. To date, little is known about the results of this investigation.
- The Wyeth company patented biphenyl-substituted pulvinones in 2005 because of their very good activity against Gram-positive bacteria. However, until now, antibiotics based on the pulvinon scaffold have only been used in animals.
presentation
Jacob Volhard was the first to synthesize vulpinic acid, pulvic acid and pulvinone. To date ten total syntheses of pulvinones have been published:
- 1895 by Claisen and Ewan,
- 1975 and 1979 by Knight and Pattenden,
- 1979 by Jerris, Wovkulich and Amos B. Smith III,
- 1984 by Ramage et al. ,
- 1985 by Campbell et al. ,
- 1990 by Gill et al. ,
- 2005 by Caufield et al. ,
- 2006 by Antane et al. ,
- 2007 by Kaczybura and Brückner,
- 2007 by Bernier, Moser and Brückner.
Individual evidence
- ↑ a b R. Ramage, GJ Griffiths, FE Shutt, JNA Sweeney, J. Chem. Soc., Perkin Trans. 1 , 1984 , 1539-1545. doi: 10.1039 / P19840001539 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Canstatt's Annual Report on Advances in Pharmacy and Allied Sciences in All Countries , Harvard University, Volume 10 (1861).
- ↑ A. Spiegel, Ber. German Chem. Ges. , 1880 , 13 , 2, 1629-1635. doi: 10.1002 / cber.18800130293 .
- ↑ A. Spiegel, Ber. German Chem. Ges. , 1880 , 13 , 2, 2219-2221. doi: 10.1002 / cber.188001302237 .
- ↑ A. Spiegel, Ber. German Chem. Ges. , 1881 , 14 , 1, 873-874. doi: 10.1002 / cber.188101401183 .
- ↑ a b A. Spiegel, Ber. German Chem. Ges. , 1881 , 14 , 2, 1686-1696. doi: 10.1002 / cber.18810140230 .
- ↑ a b c J. Volhard, Annal. Chem. , 1894 , 282 , 1-21. doi: 10.1002 / jlac.18942820102 .
- ↑ a b L. Claisen, Th. Ewan, Annal. Chem. , 1895 , 284 , 245-299. doi: 10.1002 / jlac.18952840302 .
- ↑ RL Edwards, M. Gill, J. Chem. Soc., Perkin Trans. 1 , 1973 , 1921-1929. doi: 10.1039 / P19730001921 .
- ↑ N. Ojima, S. Takenaka, S. Seto, Phytochemistry , 1973 , 12 , 2527-2529.
- ↑ N. Ojima, K. Ogura, S. Seto, J. Chem. Soc., Chem. Commun. , 1975 , 717-718.
- ↑ N. Ojima, S. Takenaka, S. Seto, Phytochemistry , 1975 , 14 , 573-576.
- ↑ N. Ojima, I. Takahashi, K. Ogura, S. Seto, Tetrahedron Lett. , 1976 , 17 , 1013-1014.
- ↑ I. Takahashi, N. Ojima, K. Ogura, S. Seto, Biochemistry , 1978 , 17 , 2696-2702.
- ↑ M. Kobayashi, N. Ojima, K. Ogura, S. Seto, Chem. Lett. , 1979 , 579-582.
- ↑ H. Sugiyama, N. Ojima, M. Kobayashi, Y. Senda, J. Ishiyama, S. Seto, Agric. Biol. Chem. , 1979 , 43 , 403-404.
- ↑ B. Steffan, W. Steglich, Angew. Chem., Int. Ed. , 1984 , 23, 6, 445-447. doi: 10.1002 / anie.198404451 .
- ↑ Gill, M., Lally DA, Phytochemistry , 1985 , 24 , 1351-1354. doi: 10.1016 / S0031-9422 (00) 81131-0 .
- ↑ T. Le Gall, C. Mioskowski, B. Amekraz et al. Angew. Chem., Int. Ed. , 2003 , 42, 11, 1289-1293. doi: 10.1002 / anie.198404451 .
- ↑ D. Klostermeyer, L. Knops, T. Sindlinger, K. Polborn, W. Steglich Eur. J. Org. Chem. , 2000 , 4, 603-609. doi: 10.1002 / anie.200390332 .
- ↑ a b M. Gill, W. Steglich, Prog. Chem. Org. Nat. Prod. , 1987 , 51 , 1-317. Springer publishing house .
- ↑ K. Rehse, J. Wagenknecht, N. Rietbrock, Arch. Pharm. , 1978 , 311 , 986-991.
- ↑ K. Rehse, U. Emisch, Arch. Pharm. , 1982 , 315 , 1020-1025.
- ↑ K. Rehse, J. Schinke, G. Bochert, Arch. Pharm. , 1979 , 312 , 390-394.
- ↑ K. Rehse, J. Lehmke, Arch. Pharm. , 1985 , 318 , 11-14.
- ↑ a b A.C. Campbell, MS Maidment, JH Pick, DFM Stevenson, J. Chem. Soc., Perkin Trans. 1 , 1985 , 1567-1576. doi: 10.1039 / P19850001567 .
- ^ A b C. E. Caufield, SA Antane, KM Morris, SM Naughton, DA Quagliato, PM Andrae, A. Enos, JF Chiarello, J. (Wyeth, and Brother Ltd., USA), WO 2005019196 , US 2005054721 , 2005 .
- ↑ a b S. A. Antane, CE Caufield, W. Hu, D. Keeney, P. Labthavikul, KM Morris, SM Naughton, PJ Petersen, SA Rasmussen, G. Singh, Y. Yang, Bioorg. Med. Chem. Lett. , 2006 , 176-180. doi: 10.1016 / j.bmcl.2005.09.021 .
- ↑ DW Knight, G. Pattenden, J. Chem. Soc., Chem. Commun. , 1975 , 876-877. doi: 10.1039 / C39750000876 .
- ↑ DW Knight, G. Pattenden, J. Chem. Soc., Perkin Trans. 1 , 1979 , 70-76. doi: 10.1039 / P19790000070 .
- ↑ PJ Jerris, PM Wovkulich, AB Smith III, Tetrahedron Lett. , 1979 , 20, 4517-4520. doi: 10.1016 / S0040-4039 (01) 86637-5 .
- ↑ M. Gill, MJ Kiefel, DA Lally, A. Ten, Aust. J. Chem. 1990 , 43 , 1497-1518.
- ↑ N. Kaczybura, R. Brückner, Synthesis , 2007 , 118-130. doi: 10.1055 / s-2006-950378 .
- ↑ D. Bernier, F. Moser, R. Brückner, Synthesis , 2007 , 15, 2240-2248. doi: 10.1055 / s-2007-983800 .
- ↑ D. Bernier, R. Brückner, Synthesis , 2007 , 15, 2249-2272. doi: 10.1055 / s-2007-983803 .


