Radio protector
A radio protector is a drug that, once administered , is intended to selectively protect healthy cells from the toxic effects of ionizing radiation . This protective function allows the radiation dose to be increased in radiation therapy directed against malignant tumors ( cancer ) in order to increase the effectiveness of radiation therapy. Radio protectors are a subgroup of radio modulators .
description
With conventional radiation therapy (radiation), the low sensitivity of many tumors to radiation - compared to the surrounding healthy tissue - is one of the greatest problems. In order to safely kill as many tumor cells as possible, a significantly higher radiation dose would be necessary in many cases. However, this is usually not possible because of the sensitivity of the surrounding healthy tissue to ionizing radiation. In radiation therapy, two different pharmacological approaches are therefore pursued in order to destroy as many tumor cells as possible and to damage as few healthy cells as possible. One of them are radiosensitizers , which are supposed to increase the sensitivity of tumor cells to radiation. Another are radio protectors, which are supposed to protect healthy cells from radiation. The ionizing radiation, for example X-rays or gamma rays , creates highly toxic free radicals in the affected cells , which in turn lead to reactive oxygen (ROS) and nitrogen species (RNS). These highly reactive species are essentially responsible for the effectiveness of radiation therapy. They lead to irreparable damage, for example double strand breaks , to the DNA in the nucleus of a cell . Radioprotectors are supposed to prevent this damage as selectively as possible, i.e. only in healthy cells.
A large number of different substances have been developed for the purpose of intracellular radio protection . They should intercept the free radicals generated in the cells and thereby reduce the rate of electron withdrawal. This anti-oxidative effect reduces the oxygen effect. To be able to develop this effect, the radio protectors must be administered before the irradiation and must be inside the cells. This is the only way for the molecules of the radio protectors to diffuse sufficiently quickly into the areas in which they can meet the appropriate reaction partners - free radicals and reactive oxygen or nitrogen species - and render them harmless. Another mechanism of action in the form of a repair process is the release of electrons to the defective bonds in the DNA. This is only possible with reducing agents ( electron donors ).
Drug classes
Compounds with free thiol groups (thiols), or prodrugs , which release the corresponding thiols after metabolism (metabolism) are primarily used as radio protectors . An example of a natural radio protector is the pseudotripeptide glutathione , which protects against free radicals and ROS inside the cell. However, glutathione is less suitable as an externally supplied radio protector , as it is broken down into its three amino acid components cysteine , glutamic acid and glycine before being transported into the cells . Other natural radio protectors are the amino acid cysteine (a component of glutathione) and its metabolic product, the biogenic amine cysteamine .
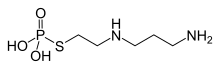
The active ingredient amifostine , a phosphorylated aminothiol , is explicitly approved as a drug for radio protection. This prodrug is broken down in the cells by alkaline phosphatases into the actual active ingredient 2 - ((aminopropyl) amino) ethanethiol . The selective effect of amifostine is obviously caused by the higher activity of the alkaline phosphatases, the comparatively higher pH value and the more favorable permeation behavior of healthy tissue. It is usually given as a short fifteen-minute infusion about half an hour before a radiation session.
In addition to the thiols, with the approved active ingredient amifostine, a number of other radio protectors are in (pre) clinical development . These include, for example, radical scavengers such as TEMPOL (a 4-hydroxy derivative of the free radical 2,2,6,6-tetramethylpiperidinyloxyl ), cytokines such as interleukin-1β , stem cell factor , enzymes such as superoxide dismutase (SOD), various vasoconstrictors , cimetidine , serotonin , Melatonin , pyridoxine , sodium selenite and sodium tungstate .
The effectiveness of a radio protector can be measured in model organisms with the help of electron spin resonance (ESR). The signal attenuation of the spin label 3-Carbamoyl-PROXYL (3-Carbamoyloxy-2,2,5,5-tetramethyl-pyrrolidine-N-oxyl, CAS # 4399-80-8) is measured. One hour after the X-ray exposure, the 3-carbamoyl-PROXYL is administered as a spin probe . If a radio protector was administered before the irradiation, the ESR signal is more attenuated. Another, more established method is to use a comet assay .
Medical history
The first work on radio protectors began as part of the Manhattan Project .
literature
- D. Citrin, AP Cotrim, F. Hyodo, BJ Baum, MC Krishna, JB Mitchell: Radioprotectors and mitigators of radiation-induced normal tissue injury. In: Oncologist Volume 15, Number 4, 2010, pp. 360-371, ISSN 1549-490X . doi : 10.1634 / theoncologist.2009-S104 . PMID 20413641 . PMC 3076305 (free full text). (Review).
- SJ Hosseinimehr: Trends in the development of radioprotective agents. In: Drug Discovery Today Volume 12, Numbers 19-20, October 2007, pp. 794-805, ISSN 1359-6446 . doi : 10.1016 / j.drudis.2007.07.017 . PMID 17933679 . (Review).
- DM Brizel: Pharmacologic approaches to radiation protection. In: Journal of clinical oncology Volume 25, Number 26, September 2007, pp. 4084-4089, ISSN 1527-7755 . doi : 10.1200 / JCO.2007.11.5816 . PMID 17827457 . (Review).
- JF Fowler: Eighth annual Juan del Regato lecture. Chemical modifiers of radiosensitivity – theory and reality: a review. In: International Journal of Radiation Oncology - Biology - Physics . Volume 11, Number 4, April 1985, ISSN 0360-3016 , pp. 665-674, PMID 3884559 (review).
- TL Phillips: Chemical modifiers of cancer treatment. In: International journal of radiation oncology, biology, physics. Volume 10, Number 9, September 1984, ISSN 0360-3016 , pp. 1791-1794, PMID 6480462 .
Individual evidence
- ↑ B. Kaser-Hotz: Principles of radiation therapy. In: M. Kessler (Ed.): Small animal oncology: diagnosis and therapy of tumor diseases in dogs. Georg Thieme Verlag, 2005, ISBN 3-830-44103-7 , p. 160. Restricted preview in the Google book search.
- ^ DJ Lee, M. Moini, J. Giuliano, WH Westra: Hypoxic sensitizer and cytotoxin for head and neck cancer. In: Annals of the Academy of Medicine, Singapore. Volume 25, Number 3, May 1996, pp. 397-404, ISSN 0304-4602 , PMID 8876907 (review).
- ↑ a b H. Krieger: Fundamentals of radiation physics and radiation protection. Edition 3, Verlag Vieweg + Teubner, 2009, ISBN 3-834-80801-6 , p. Limited preview in the Google book search.
- ↑ JR Kouvaris, VE Kouloulias, LJ Vlahos: Amifostine: the first selective-target and broad-spectrum radio protector. In: The oncologist Volume 12, Number 6, June 2007, pp. 738-747, ISSN 1083-7159 . doi : 10.1634 / theoncologist.12-6-738 . PMID 17602063 . (Review).
- ↑ Apply the radio protector as a bolus. ( Memento of October 17, 2013 in the Internet Archive ) Praxis-Depesche 15, 1999, based on: W. Wagner, A. Radmard, G. Mansour, K. Schonekas, S. Zaknoen: Improved Feasibility of Amifostine Application in Radiotherapy by Using a New Administration Schedule (Meeting abstract). In: 1999 ASCO Annual Meeting Meeting Abstract # 2348
- ↑ a b c d e Y. Miura, K. Anzai, J. Ueda, T. Ozawa: Novel approach to in vivo screening for radioprotective activity in whole mice: in vivo electron spin resonance study probing the redox reaction of nitroxyl. In: Journal of radiation research Volume 41, Number 2, June 2000, pp. 103-111, ISSN 0449-3060 . PMID 11037578 .
- ^ R. Neta, S. Douches, JJ Oppenheim: Interleukin 1 is a radioprotector. In: Journal of Immunology (Baltimore, Md.: 1950) Volume 136, Number 7, April 1986, pp. 2483-2485, ISSN 0022-1767 . PMID 3512714 .
- ↑ JR Maisin, C. Albert, A. Henry: Reduction of short-term radiation lethality by biological response modifiers given alone or in association with other chemical protectors. In: Radiation research Volume 135, Number 3, September 1993, pp. 332-337, ISSN 0033-7587 . PMID 8397428 .
- ↑ A. Shirazi, G. Ghobadi, M. Ghazi-Khansari: A radiobiological review on melatonin: a novel radioprotector. In: Journal of radiation research Volume 48, Number 4, July 2007, pp. 263-272, ISSN 0449-3060 . PMID 17641465 . (Review).
- ↑ D. Thotala, S. Chetyrkin, B. Hudson, D. Hallahan, P. Voziyan, E. Yazlovitskaya: Pyridoxamine protects intestinal epithelium from ionizing radiation-induced apoptosis. In: Free radical biology & medicine Volume 47, Number 6, September 2009, pp. 779-785, ISSN 1873-4596 . doi : 10.1016 / j.freeradbiomed.2009.06.020 . PMID 19540915 . PMC 2739572 (free full text).
- ↑ UM Schleicher, C. Lopez Cotarelo, D. Andreopoulos, S. Handt, J. Ammon: Radioprotection of human endothelial cells by sodium selenite. In: Medical Clinic Volume 94, 1999, pp. 35-38, doi : 10.1007 / BF03042188 PMID 10554526
- ↑ BS Margulies, TA Damron, MJ Allen: The differential effects of the radioprotectant drugs amifostine and sodium selenite treatment in combination with radiation therapy on constituent bone cells, Ewing's sarcoma of bone tumor cells, and rhabdomyosarcoma tumor cells in vitro. In: Journal of orthopedic research Volume 26, Number 11, November 2008, pp. 1512-1519, ISSN 1554-527X . doi : 10.1002 / jor.20679 . PMID 18473385 .
- ↑ K. Sato, M. Ichimasa, K. Miyahara, M. Shiomi, Y. Nishimura, Y. Ichimasa: Radioprotective effects of sodium tungstate on hematopoietic injury by exposure to 60Co gamma-rays in Wistar rats. In: Journal of radiation research Volume 40, Number 2, June 1999, pp. 101-113, ISSN 0449-3060 . PMID 10494142 .
- ↑ AC Müller: Valence of the Comet Assay for the detection of radio protection. (PDF; 1.6 MB) Dissertation, Martin Luther University Halle-Wittenberg, 2004
- ^ CK Nair, DK Parida, T. Nomura: Radioprotectors in radiotherapy. In: Journal of radiation research Volume 42, Number 1, March 2001, pp. 21-37, ISSN 0449-3060 . PMID 11393887 . (Review).